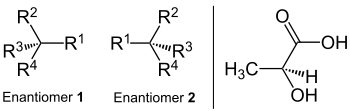
Enantiomer
Enantiomers are stereoisomers of chemical compounds, which are identical in their constitution and in the spatial structures relate to a counterpart like its (non-congruent) mirror image. Because of this, they are also called mirror image isomers . The Greek naming shows this meaning: ἐνάντιος, counterpart, and μέρος, part or area. The empirical formula and the connection of the respective atomic formations match. It is a form of configuration isomerism ; In contrast to conformational isomers , enantiomers can not be made to coincide by rotating atomic bonds. Since enantiomers in all stereocenters each have the opposite configuration, there is theoretically always one (-) - and one (+) - enantiomer, of which often only one is present in nature.
physical and chemical properties
This type of isomerism is called chirality (handedness). To illustrate the mirror image of enantiomers, body parts such as the left and right hand or everyday objects such as the left and right shoe as well as left and right turning screws or nuts can be used. An example of a molecule that occurs in two spatial variants is lactic acid in yogurt. Enantiomers always have the opposite configuration in all stereocenters. On the other hand there are the diastereomers , in which at least one stereocenter is always the same and at least one is configured differently (see there).
Enantiomers possess except the optical activity of the same physical properties as melting and boiling points, density, solubility, infrared spectra, X-ray diffraction spectra, etc. They are optically active, thus rotating the polarization plane of the linearly polarized light in a clockwise direction ( dextrorotatory form, including (+) -Form or previously called d -form) or counterclockwise ( left-rotating form, (-) - form or earlier called l -form). The sense of rotation is to be understood in relation to the viewing direction of the observer, not in relation to the beam direction. Enantiomers rotate the plane of polarization of linearly polarized light by the same amount in the opposite direction.
The two enantiomers of a starting material react differently in chemical reactions in which another enantiomerically pure reaction partner is involved. The reaction transition states are then diastereomeric to one another. Even when used as a medicinal substance in organisms, substances enantiomeric to one another produce different effects. This can be illustrated with an example from everyday life, putting on gloves: It is clear that only the right glove fits the right hand. If you try to pull the right glove onto your left hand, you will fail or only achieve a very poor result. Thus, instead of a desired effect, one achieves a useless or harmful and thus undesirable result.
Significance for the biological effect
Many biologically important substances are chiral, not only the smaller molecules of amino acids and sugars , but also biological macromolecules such as enzymes or receptors. In some substance classes, one enantiomer often predominates, for example almost all natural amino acids are in the L form. The D -form is in the natural sugars (e.g., D - glucose ) very dominant, L sugars are rare exotics. Chirality, as a result of the spatial structure of molecules , is of crucial importance for the functioning of biological systems, which are all themselves chiral. Many enzyme reactions are specialized in one enantiomer, either the levorotatory or the dextrorotatory : The reaction speed with the mirror-image enantiomer as substrate is significantly slower or it is not converted at all, since the active center of an enzyme can often take up one enantiomer more easily than that others ( lock and key principle , substrate specificity ).
The substrate specificity can be illustrated by the three-point interaction concept for enantiomers, as shown in the pictorial scheme: the chiral enantiomer A is congruent to the receptor. However, the mirror image of enantiomer A, the enantiomer B, is not suitable, which can lead to binding problems and thus affect the effect of a substance (for example a drug ). There may be differences in pharmacodynamics or pharmacokinetics. The enantiomer with the higher activity or affinity is called the eutomer and the one with the lower activity or affinity is called the distomer .
It is not uncommon for the “wrong” enantiomer to develop a completely different biological effect. Examples:
- Taste: The amino acid ( S ) - valine tastes bitter, ( R ) valine tastes sweet,
- Odor: The terpene ( S ) - (+) - carvone smells like caraway, its enantiomer ( R ) - (-) - carvone smells like mint.
- Pharmacological effects of beta blockers : In beta blockers, the ( S ) enantiomer acts selectively on the heart, while the ( R ) enantiomer acts on the cell membranes of the eye. A high enantiomeric purity is therefore of great importance for many drugs.
- Pharmacological effects of thalidomide : The thalidomide scandal made the public aware of the teratogenic effects of thalidomide. This was associated early on with the different effects of the two enantiomers of one and the same substance, since the ( S ) -enantiomer of thalidomide alone has a teratogenic ( teratogenic ) effect, but the ( R ) -enantiomer does not.
The thalidomide enantiomers, however, have the property that they convert into one another (racemize) in the body within about eight hours. The use of ( R ) -configured thalidomide only remains meaningless in practice.
In the synthetic production of differently acting enantiomeric active ingredients, for example in pharmacology, attempts are now being made from the outset to only produce the enantiomer with the desired effect and use it as a pharmaceutical active ingredient, while the other enantiomer with its possibly undesired - up to toxic - effect want to exclude from the start ( enantioselective synthesis ). Alternatively, a racemate (1: 1 mixture of two enantiomers) can be subjected to racemate resolution in order to obtain a uniform (= enantiomerically pure) active pharmaceutical ingredient, which can then be pharmacologically active with much higher selectivity than the racemate.
The smell or taste of substances can also be different depending on the enantiomer, because the corresponding receptors in the body are always themselves chiral (more precisely: enantiomerically pure).
Enantiomers are usually metabolized differently in biological systems.
Racemate
A 1: 1 mixture of (+) - and (-) - form of a substance in which the optical activity of the individual substances is balanced out is called a racemate , e.g. B. (±) - methionine [synonyms: DL -methionine and ( RS ) -methionine]. It is not optically active and has an angle of rotation α of 0 °, since the components of the right-turning and left-turning form cancel each other out. The quotient of the measured angle of rotation to the maximum angle of rotation of the pure enantiomer multiplied by 100 gives the optical purity (%) of the enantiomer mixture. Assuming ideal behavior (no interaction between the two enantiomers and validity of the Lambert-Beer law ), the optical purity is equal to the enantiomeric excess ee .
The melting point of a racemate usually deviates from the melting point of the pure enantiomers. The melting point of the racemate can be lower or higher than that of the pure enantiomers. This phenomenon, unexpected at first glance, can be explained: If the racemate crystallizes as a racemic mixture (conglomerate), the crystals of the (+) - and (-) - form are present separately, i.e. i.e., the (+) - enantiomer has a higher affinity for (+) - molecules and the (-) - enantiomer has a higher affinity for (-) - molecules. When crystallizing, pure (+) and (-) crystals are created “next to each other”. The melting point of the “racemic mixture” is well below the melting point of the pure enantiomers. Example: Both pure (+) - and (-) - enantiomers of the drug glutethimide melt at 102–103 ° C. In contrast, (±) -glutethimide, i.e. the racemic mixture, has a melting point of 84 ° C.
The situation is different if the (+) - enantiomers preferentially combine with the (-) - enantiomers during crystallization. Then "every" crystal contains the same number of molecules of "both" enantiomers. This case is called a racemic compound . The racemic compound differs in its physical properties from the pure enantiomers. The melting point can be higher, equal to or lower than that of the pure enantiomers. Example: The pure enantiomers of the drug ibuprofen have a melting point of 50-52 ° C, racemic ibuprofen has a melting point of 75-77.5 ° C. Racemic ibuprofen thus crystallizes as a racemic compound.

R and S sequence rule (CIP rule)
- Enantiomers are classified according to the R and S sequence rule.
- To find out whether an enantiomer has the ( R ) - or ( S ) -configuration, one has to order all substituents according to their priority: 1> 2> 3> 4. The substituent with the lowest priority (4) is rotated below the level of the paper. Now you go from 1 to 2 to 3.
- If the direction in which to move, with runs clockwise, is the enantiomer ( R ) configuration (from lat. Rectus , legally, right, right ')
- If the direction in which to move, against proceeds clockwise, is the enantiomer ( S ) configuration (from lat. Sinister , left ')
- See also: Cahn-Ingold-Prelog Convention for an explanation of how to prioritize the substituents.
The clockwise direction, which results from counting the priorities of the substituents to determine the configuration [( R ) or ( S )], can not automatically determine the angle of rotation α or the direction of rotation [(+) or (-)] of the plane of polarization of the linearly polarized light getting closed. Examples:
- ( S ) - Alanine has an angle of rotation α of + 13.0 ° (c = 2 in 5 N hydrochloric acid)
- ( R ) - Cysteine has an angle of rotation α of + 7.9 ° (c = 2 in 5 N hydrochloric acid)
nomenclature
To differentiate between the enantiomers, the CIP convention (Cahn-Ingold-Prelog convention, also RS nomenclature) is used, which describes the spatial arrangement of the substituents. For certain substance classes (sugar, limited to the biochemistry also for amino acids) the older Fischer projection ( D , L -nomenclature) is still used, which has the advantage that the names of related compounds are the same. In the name of a connection, the direction of rotation of the light can be made clear by adding "(+) -" for clockwise or "(-) -" for counterclockwise; z. B. (-) - tartaric acid or (+) - lactic acid , this description is not always clear, because the solvent used can influence the direction of rotation in some cases and thus change it.
Often the prefix Levo- or Lev- (left) is used for left- rotating substances and Dex- or Dextro- (right) for right-rotating substances.
Examples:
- Levodopa , levothyroxine , levonorgestrel , levofloxacin , levobupivacaine , levetiracetam , levocetirizine
- Dextrose , dexamfetamine , dexibuprofen , dexketoprofen , dextromethorphan , dexrazoxane , dexchlorpheniramine
Derived from the CIP nomenclature, ( S ) -enantiomers of medicinal substances can have the prefix Es- and ( R ) -enantiomers the prefix Ar- if there is already a non-proprietary name for the racemic substance. Conversely, the prefix Rac- is occasionally put in front of the trivial or enantiomer name for the designation of racemates .
Examples:
- Armodafinil , arhalofenate
- Eszopiclone , esomeprazole , escitalopram , esketamine
- Racecadotril , Racepinephrine
history
In 1848 Louis Pasteur succeeded in resolving the racemate for the enantiomers of a salt of D - and L - tartaric acid . For him they only differed in that their crystals were built up in mirror image. After careful crystallization, he was able to laboriously separate the different crystals by hand and thus initiated the study of enantiomerism. Tartaric acid also played an important role in the merging of the optical activity of a substance and the absolute configuration of the molecules by Johannes Martin Bijvoet . Sodium rubidium tartrate (a salt of tartaric acid) played a central role in reliably elucidating the absolute configuration of enantiomerically pure molecules. The discoverers were awarded the Nobel Prize in Chemistry for this.
chemistry
Synthetic chemistry now has methods for the direct, targeted production of a pure active ingredient isomer through enantioselective or even enantiospecific syntheses based on the model of nature.
Asymmetric synthesis
Chemical syntheses of chiral substances usually produce both enantiomers in the same ratio. You have to be laboriously separated in order to obtain the enantiomers as a pure substance . The synthesis of enantiomerically pure molecules is one of the most difficult fields in preparative organic chemistry. Numerous newer synthetic processes, some of which have very high enantioselectivities , offer a way out . Various methods have been developed to make a chiral molecule accessible from non-chiral starting materials:
- Use of chiral auxiliary reagents and catalysts (e.g. chiral phosphines)
- Implementation with enzymes
- Attachment of an auxiliary that can be removed again after the reaction
- Conversion into diastereomers [by adding an enantiomerically pure substituent such as (-) - strychnine ] and their separation (e.g. crystallization, column chromatography, etc.)
The enantiomeric purity achieved here is often different. The enantiomeric excess is given as a measure of the success of the asymmetric synthesis / crystallization:
In addition, the synthesis of enantiomerically pure active ingredients from chiral natural products (examples: amino acids , carbohydrates , terpenes , alkaloids , steroids ) remains an important and efficient method.
Drug synthesis
The pharmacologist Everhardus Ariëns is regarded as an important pioneer for the targeted enantiomerically pure drug synthesis , who in the 1980s complained about racemic drugs as enantiomerically contaminated active substances. With the advancement of synthetic chemistry, the stereoselective, fully synthetic production of pure enantiomers with one to a few centers of chirality is nowadays often possible without great effort. Medicinal substances with many centers of asymmetry, on the other hand, are produced using natural substances, with a few exceptions, using semi-synthetic methods.
A trend towards the synthesis of enantiomerically pure substances can be seen among the monochiral drugs. From 1999 to 2003, 15 pure enantiomers were to be found out of a total of 24 new introductions, the number rose to 20 pure enantiomers among 25 new introductions for the period from 2004 to 2008. Most of them are completely new substances, i.e. enantiomers that have no racemate precursors. In the following two 5-year periods, the proportion of enantiomerically pure new developments was still high.
However, some enantiomeric drugs have been developed to replace high-selling racemates as eutomers. However, an enantiomerically pure substance does not always offer a real therapeutic advantage over the racemate. It is doubtful whether the pharmacologically active dexibuprofen [( S ) -enantiomer of racemic ibuprofen ], introduced in 2001, is a real advance, since the ( R ) -enantiomer of ibuprofen is rapidly converted into the active ( S ) -form after absorption anyway becomes. According to the manufacturer's recommendation, both the enantiomerically pure and the racemic drug should be dosed in the same way. The therapeutic superiority of the cough blocker levodropropizine , introduced in 2000, over the use of the racemate is also questionable, as no clear distinction between an effective and an ineffective enantiomer was known and both are dosed similarly. The proton pump inhibitor esomeprazole, which was also introduced in 2000, as a prodrug of the achiral active form does not have a stronger effect than the ( R ) form of omeprazole , but is more bioavailable due to a slower enzymatic metabolism . Nevertheless, due to the mechanism of action of the proton pump inhibitors, the therapeutic relevance has been questioned.
On the other hand, the substances escitalopram , levocetirizine , levobupivacaine and dexrazoxane , for example, are seen as therapeutic progress compared to the effectiveness of the predecessor racemates .
For some substances, the center of chirality is not in the pharmacologically active molecular range, such as the gyrase inhibitors gatifloxacin or nadifloxacin . Then the use of the racemate should be justifiable.
See also
literature
- EJ Ariëns: Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology . In: European Journal of Clinical Pharmacology . tape 26 , no. 6 , 1984, pp. 663-668 , doi : 10.1007 / BF00541922 .
- Adam Sobanski, Roland Schmieder, Fritz Vögtle : Topological Stereochemistry and Chirality . In: Chemistry in Our Time . tape 34 , no. 3 , 2000, pp. 160-169 , doi : 10.1002 / 1521-3781 (200006) 34: 3 <160 :: AID-CIUZ160> 3.0.CO; 2-6 .
- Klaus Roth : A never-ending chemical story . In: Chemistry in Our Time . tape 39 , no. 3 , 2005, p. 212-217 , doi : 10.1002 / ciuz.200590038 .
- Bernard Testa: Fundamentals of Organic Stereochemistry . Wiley-VCH, 1983, ISBN 3-527-25935-X .
- Uwe Meierhenrich: Amino acids and the asymmetry of life . Springer-Verlag, 2008, ISBN 978-3-540-76885-2 .
Web links
Individual evidence
- ↑ HJ Roth, CE Müller, G. Folkers (eds.): Stereochemistry & Drugs: Basics - Consideration - Impact, Scientific Publishing Company mbH, Stuttgart, pp. 80, 81, 1998.
- ↑ Wolfgang Legrum: Fragrances, between stink and fragrance , Vieweg + Teubner Verlag (2011) pp. 33–35, ISBN 978-3-8348-1245-2 .
- ↑ Everhardus Ariëns : Stereochemistry, a basis for sophisticated nonsense in pharmacokinetics and clinical pharmacology , European Journal of Clinical Pharmacology 26 (1984) 663-668, doi : 10.1007 / BF00541922 .
- ↑ Nature (London) 385, 303 (1997).
- ↑ M. Reist, PA Carrupt, E. Francotte, B. Testa: Chiral inversion and hydrolysis of thalidomide: mechanisms and catalysis by bases and serum albumin, and chiral stability of teratogenic metabolites . In: Chemical Research in Toxicology . 11, No. 12, 1998, pp. 1521-8. doi : 10.1021 / tx9801817 . PMID 9860497 .
- ↑ Bernd Engels, Carsten jewelry, Tanja Schirmeister, Reinhold Fink: Chemistry for medical professionals . ( google.de ).
- ↑ Hermann J. Roth , Christa E. Müller and Gerd Folkers: Stereochemie & Arzneimittel , Wissenschaftliche Verlagsgesellschaft Stuttgart, 1998, ISBN 3-8047-1485-4 , pp. 161–162.
- ↑ Duden: rectus .
- ↑ a b Hans-Dieter Jakubke, Hans Jeschkeit: amino acids, peptides, proteins , Verlag Chemie, Weinheim, p. 30, 1982, ISBN 3-527-25892-2 .
- ↑ a b H. J. Roth: Dex-, Lev-, Ar-, Es-, Rac-, new "pure" drugs - balance of the last five years . In: Deutsche Apothekerzeitung . tape 149 , no. 28 , 2009, p. 3182-6 .
- ↑ HJ Roth: New Chiral Drugs - A Review of the Years 2009 to 2013 . In: Deutsche Apothekerzeitung . No. 4 , 2014 ( deutsche-apotheker-zeitung.de ).
- ↑ H. J. Roth: Chiral versus achiral drugs . In: Deutsche Apothekerzeitung . No. 6 , 2019 ( deutsche-apotheker-zeitung.de ).
- ↑ a b c H. J. Roth: Dex-, Lev-, Es-, a balance sheet for the last five years: trend towards the application of pure enantiomers . In: Deutsche Apothekerzeitung . tape 144 , no. 4 , 2004, p. 2309 .