Barber reaction
The Barbier reaction , also known as the Barbier rearrangement , is a name reaction from the field of organic chemistry . It is named after the French chemist Philippe Antoine François Barbier (1848–1922), the doctoral supervisor of Victor Grignard . It was first published in 1898, just two years before the Grignard reaction .
Overview
The Barbier reaction has very wide-ranging applications in organic chemistry, such as B. in the synthesis of secondary or tertiary alcohols . An organic radical ( alkyl , aryl , benzyl or allyl radical ) halogenated with chlorine , bromine or iodine reacts on a metallic surface made of magnesium , lithium , aluminum , samarium , antimony , bismuth , cadmium , gallium , indium , manganese , tin or zinc etc. directly in the presence of an aldehyde (R 2 , R 3 = H, organyl group ) or ketone (R 2 , R 3 = organyl group) with its carbonyl group. The hydrolysis then leads to alcohol:
A Grignard compound freshly formed from an allyl halide reacts in a side reaction with further allyl halide to form dimers. The barber response is one way to circumvent this problem:
mechanism
The mechanism is shown here using allyl bromide , propanal and metallic magnesium .
First of all, allyl bromide ( 1 ) is applied to the surface of the metallic magnesium. This creates the Grignard reagent prop-1-enmagnesium bromide ( 2 ). This is in equilibrium with an ionic and a radical form (not shown here), whereby these forms only make up a small proportion of the equilibrium. If the Grignard reagent 2 is now mixed with propanal , the hex-5-en-3-olate 3 is formed . This reacts through acidic processing to form hex-5-en-3-ol ( 4 ).
This reaction is very similar to the Grignard reaction with the difference that it is a one-pot reaction . This means that all reagents are mixed with one another at the same time; this is also known as a “one-step reaction”. In the Grignard reaction, on the other hand, the Grignard reagent must first be generated in the absence of the carbonyl substrate. The Barbier reaction falls under the group of nucleophilic addition reactions . In contrast to the Grignard reaction, it can also be used in aqueous solvents because the organometallic intermediates are insensitive to protic solvents. For this reason, the Barbier reaction can be assigned to Green Chemistry . It also has the advantage over the Grignard reaction that it gives the same results with significantly less toxic halides. It is preferably used for the reaction of allyl or benzyl bromides. It is also thought that they are using a single-electron transfer (Engl. Single-electron-transfer , SET) expires.
Examples
The following examples are intended to illustrate the broad application possibilities of the Barbier reaction. The reaction between propargyl bromide and butanal in the presence of metallic zinc gives an alkyne derivative:
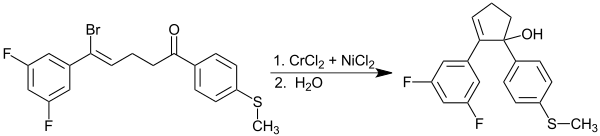
The Barbier reaction can also take place intramolecularly, as shown in the following example. In this way, correspondingly substituted molecules can be cyclized with the help of the Barbier reaction. The catalytically active nickel (0) is previously formed from chromium (II) chloride and nickel chloride through a redox reaction.
Individual evidence
- ↑ George D. Bennett, Leo A. Paquette : Allylindation in Aqueous Media: Methyl 3‐ (Hydroxymethyl) ‐4 ‐ Methyl ‐ 2 ‐ Methylenepentanoate In: Organic Syntheses . 77, 2000, p. 107, doi : 10.15227 / orgsyn.077.0107 ( PDF ).
- ^ Gary W. Breton, John H. Shugart, Christine A. Hughey, Brian P. Conrad, Suzanne M. Perala: Use of Cyclic Allylic Bromides in the Zinc-Mediated Aqueous Barbier-Grignard Reaction . In: Molecules . 6, 2001, pp. 655-662. doi : 10.3390 / 60800655 .
- ↑ J. Clayden, N. Greeves, S. Warren, P. Wothers: Organic Chemistry ; Oxford University Press, Oxford 2001, 1st edition; ISBN 978-0-19-850346-0 .
- ↑ a b c d e f Z. Wang: Comprehensive Organic Name Reactions and Reagents, 1 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, pp. 202-209, ISBN 978-0-471-70450-8 .
- ↑ László Kürti , Barbara Czakó .: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms . Elsevier Academic Press, 2005, ISBN 978-0-12-429785-2 , pp. 38-39.
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren, Peter Wothers: Organic Chemistry . Oxford University Press, 2001, ISBN 978-0-19-850346-0 , p. 224.
- ^ P. Barbier: Synthèse du diéthylhepténol . In: Compt. Rend. . 128, 1899, p. 110.
- ^ Gary W. Breton, John H. Shugart, Christine A. Hughey, Brian P. Conrad, Suzanne M. Perala: Use of Cyclic Allylic Bromides in the Zinc-Mediated Aqueous Barbier-Grignard Reaction . In: Molecules . 6, 2001, pp. 655-662. doi : 10.3390 / 60800655 .
- ↑ Christian Bernlind, Stefan Oscarson: Synthesis of a Branched Heptose- and Kdo-Containing Common Tetrasaccharide Core Structure of Haemophilus influenzae Lipopolysaccharides via a 1,6-Anhydro-l-glycero-β-d-manno-heptopyranose Intermediate . In: The Journal of Organic Chemistry . tape 63 , no. 22 , 1998, pp. 7780-7788 , doi : 10.1021 / jo9808573 .
- ↑ Artur Jõgi, Uno Mäeorg: Zn Mediated Regioselective Barbier Reaction of Propargylic Bromides in THF / aq. NH4Cl Solution . In: Molecules . 6, 2001, pp. 964-968. doi : 10.3390 / 61200964 .
- ↑ Barry M. Trost, Anthony B. Pinkerton: Enhanced geometrical control in a Ru-catalyzed three component coupling. In: Tetrahedron Letters. 41, No. 49, 2000, pp. 9627-9631, doi: 10.1016 / S0040-4039 (00) 01735-4 .