Benfluorex
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
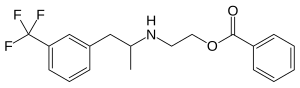
|
||||||||||||||||||||||
| Structural formula without stereochemistry | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Benfluorex | |||||||||||||||||||||
| other names |
( RS ) -2 - ({1- [3- (trifluoromethyl) phenyl] propan-2-yl} amino) ethyl benzoate |
|||||||||||||||||||||
| Molecular formula | C 19 H 20 F 3 NO 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 351.4 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Benfluorex is a drug from the group of phenylalkylamines that has been approved as a lipid lowering agent for reducing blood lipid levels and for weight loss in type II diabetes mellitus in some countries, such as France , Spain and Italy , but not in Germany . In addition, Benfluorex has been widely used as an appetite suppressant . Due to an increased incidence of heart valve damage, Benfluorex was recalled in 2009 at the instigation of the European Medicines Agency . In France alone, at least 500 deaths are seen in a possible connection with the benfluorex-containing drug Mediator . These deaths as well as the behavior of the French drug agency Agence française de sécurité sanitaire des produits de santé and the manufacturer Servier led to the so-called mediator scandal .
Clinical information
Application areas (indications)
Benfluorex was originally approved as a lipid-lowering drug for the treatment of hypertriglyceridemia and for weight reduction in type II diabetes mellitus in France and other countries. Outside of the indication, benfluorex was often used as an appetite suppressant in non-type II diabetics. At the latest with the assessment of the European Medicines Agency there is no longer any medical indication for the use of Benfluorex.
Adverse effects (side effects)
When using Benfluorex, non-specific side effects of the gastrointestinal system, such as nausea, vomiting, stomach pain and diarrhea, as well as fatigue, weakness and dizziness, were observed. In very rare cases, allergic reactions, shock, hypotension , rash, hives and Quincke's edema occurred. In addition, benfluorex was associated with cases of increased liver enzyme levels and cases of orientation and perception disorders.
Benfluorex has been associated with an increased incidence of heart valve damage and an increased incidence of pulmonary hypertension and fibrosis of the heart . According to analysis by the French Ministry of Health, the drug may have been responsible for the death of at least 500 of the approximately 5 million French patients treated with the drug Mediator. Other estimates put up to 2000 deaths in France.
Pharmacological properties
Mechanism of action (pharmacodynamics)
In its mode of action, Benfluorex is similar to fenfluramine, which was previously used as an appetite suppressant and has been withdrawn from the market due to heart valve damage and the occurrence of pulmonary hypertension . Benfluorex acts as a prodrug in the human body that works after metabolism to the active metabolite norfenfluramine . On the one hand, this metabolite increases the release of serotonin into the extracellular space and, on the other hand, acts as an agonist on various serotonin receptors , including 5-HT 2C and 5-HT 2B . While stimulation of 5-HT 2C receptors is associated with the appetite suppressing effect, changes in the heart and pulmonary vessels, which can lead to fibrosis, heart valve damage and pulmonary hypertension, can be attributed to activation of 5-HT 2B - Receptors are returned.
Development of new medicines
In the 1960s, the pharmaceutical company Servier was looking for new drugs based on the appetite suppressing amphetamine . This project led to the drugs fenfluramine and benfluorex, among others. In 1976, Benfluorex was launched in France under the name Mediator. It was also used in other European, Asian and African countries.
Undesirable side effects, suspicious transaction reports
In the 1980s and 1990s, fenfluramine, in contrast to benfluorex, came under increasing criticism due to severe side effects, including heart valve damage and pulmonary hypertension. The fenfluramine metabolite norfenfluramine, which was later identified as the cause of these side effects, was also known as a major metabolite of benfluorex at the time. While fenfluramine was withdrawn from the market in 1997 because of these serious side effects, Mediator continued to be marketed in France after its approval was suspended for two months. After the recall of fenfluramine, Mediator was increasingly used as an appetite suppressant outside of the approval. In 1998 the French Medicines Agency ordered a monitoring program for mediators. Thereafter, cases of aortic regurgitation and pulmonary hypertension have been attributed to the drug . In 2003, the active ingredient was first brought into direct association with heart valve damage.
In the years that followed, there were more suspicious reports that benfluorex, as previously observed for fenfluramine, can cause heart valve damage. In order to avoid a momentous revocation of approval, Servier waived the due reauthorisation of Mediator in Spain and Italy in 2003. It was never allowed in Germany. As a result of a surveillance program, the French Medicines Agency restricted the indications for Mediator in 2007. From the following year on, the pulmonologist Irène Frachon pointed out several times and clearly about serious side effects that she had observed in patients and reported to the authorities. However, this ignored such references. Frachon also turned to the media. In 2010 she published the book Mediator 150 mg: Combien de morts? ("How many dead?") In 2009, on the recommendation of the European Medicines Agency, the French Medicines Agency withdrew from the market. The following year, the European Medicines Agency finally revoked Mediator's approval.
Legal proceedings
In September 2019, the criminal case began in the court of justice in the Paris Palace of Justice. The charges of negligent homicide and assault are directed against 14 people and eleven institutions, including the National Agency for Drug Safety. 120 witnesses were summoned, 376 lawyers and 4981 accessory prosecutors were admitted. A judgment is expected in April 2020 at the earliest.
From 1976 until the end of approval in 2009, around five million people took the drug; Servier achieved a turnover of around 500 million euros. Over 4000 patients suffered severe damage; around 2000 had to be operated on. The number of people who died as a result of the drug is estimated at 1,500 to 2,100.
At the insistence of the French government, Servier set up a compensation fund from which those affected and their relatives have received 135 million euros so far. Initially, the company resisted payments. It was only late in admitting a connection between taking the drug and the damage it suffered.
literature
- A. Mullard: Mediator scandal rocks French medical community . In: Lancet . tape 377 , no. 9769 , March 2011, p. 890-892 , PMID 21409784 .
- I. Frachon: Mediator 150 mg: Combien de morts? édition Dialogues, coll. Ouvertures, Brest 2010, ISBN 978-2-918135-14-2 .
Movie
- La Fille de Brest (2016)
Individual evidence
- ↑ a b c d data sheet Benfluorex hydrochloride from Sigma-Aldrich , accessed on October 10, 2016 ( PDF ).
- ↑ a b French judges probe maker of discredited pill. Reuters, September 21, 2011, accessed November 10, 2011 .
- ↑ K. Boutet, I. Frachon, Y. Jobic et al: Fenfluramine -like cardiovascular side-effects of benfluorex . In: Eur. Respir. J. Band 33 , no. 3 , March 2009, p. 684-688 , doi : 10.1183 / 09031936.00086308 , PMID 19251806 .
- ↑ LW Fitzgerald, TC Burn, BS Brown et al .: Possible role of valvular serotonin 5-HT (2B) receptors in the cardiopathy associated with fenfluramine . In: Mol. Pharmacol. tape 57 , no. 1 , January 2000, p. 75-81 , PMID 10617681 .
- ↑ RB Rothman, MH Baumann, JE Savage et al: Evidence for possible involvement of 5-HT (2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications . In: Circulation . tape 102 , no. 23 December 2000, pp. 2836-2841 , PMID 11104741 .
- ^ BH Gordon: The pharmacokinetics of the metabolites of benfluorex in chronic administration [...] in human volunteers Servier Report No. 93-5792-001. 1993. (lefigaro.fr , PDF; 1.2 MB).
- ↑ Eric Favereau: Mediator, coupe-faim dangereux et longtemps toléré . In: Liberation . November 16, 2010 ( liberation.fr ).
- ^ J. Rafel Ribera, R. Casañas Muñoz, N. Anguera Ferrando, N., Batalla Sahún, A. Castro Cels, R. Pujadas Capmany: Valvular heart disease associated with benfluorex . In: Rev Esp Cardiol . tape 56 , no. 2 , February 2003, p. 215-216 , PMID 12605770 (Spanish, revespcardiol.org [PDF]).
- ^ R. Klingsieck: Doctor uncovered scandal. A mega-trial is taking place in France against the pharmaceutical company Servier and the drug supervisory authority. In: New Germany. October 1, 2019, p. 7. (neue-deutschland.de)
- ^ R. Balmer: French drug scandal in court. September 24, 2019. (nzz.ch)