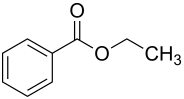
Ethyl benzoate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethyl benzoate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 10 O 2 | |||||||||||||||
| Brief description |
colorless liquid with a fruity odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 150.18 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.05 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−35 ° C |
|||||||||||||||
| boiling point |
213 ° C |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
very sparingly soluble in water (0.72 g l −1 at 25 ° C) |
|||||||||||||||
| Refractive index |
1.5007 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethyl benzoate is a chemical compound from the group of carboxylic acid esters .
It is a colorless, oily liquid with a characteristic fruity odor, which is formed as an ester from aromatic benzoic acid when it reacts with ethanol . It is a fragrance and flavoring substance .
presentation
A simple way of preparing ethyl benzoate 2 , which is often used in laboratories, is the acid-catalyzed esterification of benzoic acid 1 with ethanol:
Alternatively, benzoyl chloride can be reacted with ethanol and a base such as pyridine , triethylamine or sodium hydroxide solution .
properties
Ethyl benzoate forms flammable vapor-air mixtures. The compound has a flash point of 88 ° C. The lower explosion limit is 1 vol.% (62 g · m −3 ). The ignition temperature is 490 ° C. The substance therefore falls into temperature class T1. The electrical conductivity is rather low at 4.9 · 10 −7 S · m −1 .
Individual evidence
- ↑ a b c d e f g h i j k l m n o Entry on ethyl benzoate in the GESTIS substance database of the IFA , accessed on January 24, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-238.
- ↑ Arthur Israel Vogel. Rev. by Brian S. Furniss: Vogel's textbook of practical organic chemistry . 5th edition. Longman, Harlow 1989, ISBN 0-582-46236-3 , pp. 1076 ( limited preview in Google Book search).
- ↑ a b E. Brandes, W. Möller: Safety-related parameters. Volume 1: Flammable Liquids and Gases. Wirtschaftsverlag NW - Verlag für neue Wissenschaft, Bremerhaven 2003.
- ↑ Technical rules for hazardous substances TRGS 727, BG RCI leaflet T033 Avoidance of ignition hazards due to electrostatic charges , status August 2016, Jedermann-Verlag Heidelberg, ISBN 978-3-86825-103-6 , ISBN 978-3-86825-103-6 .

