Dipeptidyl peptidase chemistry 4
The enzyme dipeptidyl peptidase 4 ( DPP 4 , DP IV or DPP IV for short ) was first described by Hopsu-Havu and Glenner in 1964/66. The authors called the newly found enzyme dipeptide naphthylamidase. Some time later the enzyme was rediscovered by Schulz and Barth independently of Hopsu-Havu and Glenner. Ion exchange chromatography with Sephadex DEAE A-50 was decisive for enzyme purification. As a result of various investigations into the chemistry, the enzyme was named dipeptidyl peptidase IV [DP IV]. In the course of further investigations it was established for the first time that it is a serine protease. It was only in the course of time that it was recognized that this enzyme performs essential functions in the human and animal organism.
After eating, various hormones are released in the stomach, so-called incretins , which increase the release of insulin in the body. The DPP 4 splits these incretins and thus stops their effect. It follows that inhibitors of DPP 4 are potential drugs against type II diabetes mellitus , since an increased release of insulin can help to lower the chronically high blood sugar . As a result of research into dipeptidyl peptidase-4 and its inhibitors, two drugs, sitagliptin and vildagliptin , have already been approved by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency as therapeutic agents for the treatment of type 2 diabetes mellitus.
Inhibiting this enzyme would have no effect against type I diabetes mellitus : Here, no insulin can be produced at all because the islet cells of the pancreas are completely destroyed.
The functional mechanism from a chemical point of view (chemistry)
The enzyme DPP 4 occurs ubiquitously in the human, but also in the animal organism. It is a proteinase that splits off two groups of amino acids (dipeptides) from the N -terminal end of a peptide . Since the amino acid serine is located in the active center of the enzyme , it belongs to the serine proteinases . It can only split off dipeptides from the amino group end ( N terminus) of a peptide, but not from the carboxy group end, the C terminus (see peptide bond ).
An exemplary peptide as a substrate for DPP 4 looks as follows, where Xaa2, Xaa1 and X'aa1 stand for any amino acids and the vertical line for the point at which the enzyme cleaves the peptide bond. The positions of the amino acids relative to the cleavage site are described by P2, P1 and P'1:
N-Xaa2-Xaa1-|-X'aa1-… P2 P1 P'1
N-Xaa2-Xaa1-C + N-X'aa1-…
The DPP 4 preferentially cleaves peptides if a proline residue (Pro) is in the P1 position.
The substrate specificity in P1 position (Xaa1)
In addition to peptides with proline in the P1 position, DPP 4 also cleaves peptides with other amino acids in this position.
First of all, it was found that, in addition to proline, hydroxyproline and dehydroproline residues are very well accepted. Substrates with pipecolic acid residues (Pip) in the P1 position are also similarly active. In scheme 1 a “proline family tree” is presented which leads directly from proline to glycine . As an experiment, the individual di- peptide-para-nitroanilides ( pNA ) in the P1 position were exchanged for the aminoacyl residues listed in the “family tree”. It could be shown that all of these derivatives are more or less active substrates. Particularly Xaa1 = Thz, Oxa, pipecolic acid (Pip) and azetidine-2-carboxylic acid (Aze) were successfully tested in Ala-Xaa1-pNA .
Furthermore were tested Xaa1- the residues of glycine (Gly), 2-aminobutyric acid (Abu): from norvaline (Nva), sarcosine (Sar), N -ethyl-glycine, N (n-propyl) glycine, - N -methyl -2-aminobutanoic acid, N -methyl-alanine and N -ethyl-alanine. It is interesting that there is a gradation in the following direction with regard to the effectiveness of the DPP 4 substrates:
- Proline> alanine> glycine.
With regard to the kcat / KM value, the Aze derivative is comparable to the corresponding Pro-substrate. In one case it is a five-ring, in the other a four-ring. The testing of the compounds Ala-Xaa1-pNA with Xaa1 = a-5MeOxa, and s-5MeOxa was also interesting. Ala- (s-5MeOxa) -pNA acted as substrate, Ala- (a-5MeOxa) -pNA not [s = syn, a = anti]. The proline ring is not built planar, but is in a configuration analogous to Scheme 2 (left).
As a result, the positions in the ring system of proline must also be assessed differently. This explains why Xaa2-Hyp-pNA is a substrate, but Xaa2- (a-5MeOxa) -pNA is not enzymatically hydrolyzed by DPP 4.
It is observed that in substrates of the type Xaa2-Pro-X'aa1 the rate of cleavage depends on the nature of the residue X'aa1. In this case, the rate-determining step (see below) is obviously shifted from the deacylation (k3) to the preceding steps. A hindrance to the flow of these substrates into the active center of the DPP 4 is conceivable. The slowest step here should possibly be before the actual catalytic process. In the simplest case (assuming ka is much smaller than k3 and k3 is very much smaller than k2 and k1)
- kcat = k3ka and M = k-ak3 / kak1
ka = rate constant of the inflow of the substrate into the active center, ka = rate constant of the outflow of the substrate from the active center, k1 = rate constant of the tetrahedral intermediate, k2 = rate constant of the acylation step (TI1 ® acylenzyme), k3 = rate constant of the deacylation step (dipeptide ).
The substrate specificity in P2 position (Xaa2)
The importance of the N-terminal amino acid residue in position P2 (Xaa2) was also determined. The residues of: Pro, Abu, Leu, Val, Ala, Ile, Glu, Phe, Tyr, Ser, Gln, Lys, Asp, Asn as well as N, N-dimethyl-glycine were examined in Xaa2-Pro-pNA [( N, N) -DMG], N, N, N-trimethyl-glycine [(N, N, N) -TMG] and S, S-dimethyl-sulfonium-acetic acid [(S, S) -DMS]. All substances acted as substrates for DPP 4 (see Table 1).
| X-Pro- p -nitroanilid X = |
k cat in s −1 | K m in 10 5 m |
|---|---|---|
| Per | 51.5 ± 0.3 | 0.92 ± 0.03 |
| α -Aba | 72.7 ± 0.7 | 1.53 ± 0.05 |
| Leu | 65.4 ± 0.6 | 44.9 ± 0.9 |
| Val | 1.75 ± 0.04 | 1.28 ± 0.08 |
| Ala | 54.6 ± 0.4 | 1.66 ± 0.05 |
| Ile | 28.5 ± 4.9 | 39.5 ± 0.3 |
| Glu | 1.23 ± 0.67 | 2.10 ± 0.07 |
| Phe | 71.3 ± 1.7 | 4.27 ± 0.25 |
| Tyr | 62.9 ± 2.2 | 4.03 ± 0.34 |
| Ser | 61.0 ± 0.8 | 3.99 ± 0.24 |
| Gln | 69.8 ± 4.7 | 4.90 ± 0.98 |
| Lys | 54.8 ± 1.2 | 5.16 ± 0.25 |
| Gly | 78.4 ± 0.7 | 10.2 ± 0.52 |
| Asp | 30.1 ± 0.3 | 5.85 ± 0.22 |
| Asn | 51.4 ± 1.0 | 11.8 ± 0.83 |
| Sar | 97.3 ± 0.02 | 13.0 ± 0.12 |
| ( N , N ) -DMG | 0.9 ± 0.02 | 145 ± 10 |
| ( N , N , N ) -TMG | 1.5 ± 0.2 | 7040 ± 1200 |
| ( S , S ) -DMS | 0.3 ± 0.02 | 1135 ± 80 |
How far away can the N-terminal amino function be from the peptide bond between P1 and P2? For this purpose, amino acid residues of Ala, β -Ala, γ -Abu and ε -Ahx were used in Xaa2-Pro-pNA . The derivatives of Ala, β -Ala and γ -Abu are substrates of DPP 4 with a falling tendency. The ε -Ahx compound is not hydrolyzed
| X-Pro- p -nitroanilid X = |
k cat in s −1 | K m · 10 5 in M |
|---|---|---|
| Ala | 54.6 ± 0.4 | 1.66 ± 0.05 |
| β- Ala | 6.7 ± 0.1 | 154 ± 8.5 |
| γ -Abu | 0.9 ± 0.03 | 352 ± 30 |
| ε -Ahx | no hydrolytic activity | |
The role of the aminoacyl residues in the P'1 position (X'aa1)
In order to clarify the meaning of the aminoacyl residues in the P'1 position, kinetic studies were carried out with the tripeptides Ala-Pro-X'aa1. Surprisingly, it turns out that no enzymatic hydrolysis takes place if X'aa1 = Pro, Hyp, Dehydroprolyl, Pipecolyl, Aciridyl residues or in general the peptide bond to be cleaved -CO-NH- by -CO-N (R) - is replaced (R not equal to H).
The "recognition structure" of the substrates of the DPP 4
Due to the extensive kinetic data material, intensive studies of the computer simulation were carried out. One result of these efforts was the formulation of a structure that all substrates must have in order to be recognized as substrates in the active center of the DPP 4. This “recognition structure” is shown in Figure 1.
The internal H-bridge is important. Any hindrance to their training leads to inactivity as a substrate. The fact that every substitution on the NH group of the peptide bond to be cleaved prevents this, is the reason why all peptides with the structural feature -CO-N (R) - with R not equal to H are not accessible to enzymatic hydrolysis. It is to be expected that the internal H-bridge will be prevented by:
- a cis peptide bond and
- the optical antipodes
It is known that proline peptides have a measurable cis configuration on the peptide bond to be cleaved. We determined the ratio of trans to cis bond using the example of the substrate Ala-Pro-pNA and followed the enzymatic hydrolysis with the aid of fast kinetics (“ stopped-flow method ”). There is a biphasic conversion after time. In the first phase, a faster degradation, depending on the enzyme concentration, can be observed up to the percentage that was found for the presence of the trans-peptide bond, then a slow reaction course, which is determined by the enzyme-independent cis-trans- Conversion speed. Accordingly, as expected, cleavage takes place only via the trans bond, the cis bond is inactive.
The stereo specificity
Furthermore, the substrates in the P1 position are absolutely stereospecific. Ala- L- Pro-pNA e.g. B. is a good substrate, Ala- D- Pro-pNA is not enzymatically hydrolyzed. In the case of compounds of the D -Xaa2-Pro-pNA type, hydrolysis also does not take place. D -Xaa2-Ala-pNA, however, are substrates of DPP 4. Table 3 demonstrates this using the example of Ala-Ala-pNA, D -Ala-Ala.pNA and Aib-Ala-pNA. The phenomenon that D -Phe-Pro-pNA and D- Tyr-Pro-pNA are uncompetitive inhibitors of hydrolysis of Ala-Pro-pNA (the Ki values are: 0.35 ± 0.04 mM and 0.52 ± 0.04 mM), according to which both inhibitors are not competitive or mixed inhibitors, and with Ala-Ala-pNA as substrate, the compounds show no inhibitory effect, should be seen in connection with the theoretical conclusions discussed in this work.
| relationship | in | in | in |
|---|---|---|---|
| 0.92 ± 0.13 | 18.27 ± 1.81 | 19.95 ± 0.91 | |
| 0.86 ± 0.15 | 40.39 ± 4.28 | 47.33 ± 2.47 | |
| 0.95 ± 0.20 | 2.23 ± 0.34 | 2.38 ± 0.15 | |
| = Ala-Ala-pnitroanalinide, = D -Ala-Ala-pnitroanalinide, = Aib-Ala-pnitroanalinide | |||
It is noticeable that the stereoselectivity in P2, as Table 3 shows, does not affect the K M value, but has a serious effect on the k cat values. This effect, which is surprising, will be discussed below.
The sequence of enzyme catalysis
It is assumed that the sequence of reactions shown in Schena 3 is run through in the course of the enzyme catalysis :
According to this, a distinction must be made between an acylation process and a deacylation process. The substrate penetrates the active center (see recognition complex) and ES is formed. This leads to the tetrahedral intermediate of the acylation process TI 1 . After the first cleavage product has been split off, the addition of a water molecule results in the second tetrahedral intermediate TI 2 (deacylation process). After these cleavage, the free enzyme and the second cleavage product (dipeptide) are ultimately released. The first tetrahedral intermediate TI 1 is asymmetric. Theoretically, an S and / or an R shape can develop here. TI 2 , however, is symmetrical. No antipodal structures arise here .
First, the question arises as to where the rate-determining step is located in the substrates, whether in the area of acylation or deacylation. Investigations were therefore carried out with the substrates Ala-Ala-anilide and Ala-Pro-anilide with various substituted aryl rings at optimum pH. The Hansch approach (QSWA = quantitative-structure-effect-analysis) did not bring any correlation with the substituted derivatives of the Ala-Pro-Anilid series, but one in the Ala-Ala-Anilid series.
Substrat : Ala-Ala-NH-C6H4-R QSWA : lgkcat = 0,807s ± 0,186 R : -H, p-F, p-Cl, p-Br, p-CH3, p-OCH3, p-OC2H5, p-NO2, m-Cl, m-CH3, m-CF3, m-NO2
Substrat : Ala-Pro-NH-C6H4-R QSWA : keine Korrelation
This shows that the rate-determining step in the alanine series lies in the acylation, i.e. in an area where the substituted anilines have not yet been split off (k2). In the proline series, on the other hand, the rate-determining step should be the deacylation (k3). The measurements were carried out at the pH optimum (see below).
The question now is: can the conformation of the tetrahedral intermediate TI 1 be determined? For this purpose a QCAR analysis (Quantitative Conformation Activity Relationships) was carried out in the alanine series. The results suggest that the effects occur when the -NH-Ar residue emerges from the tetrahedral intermediate TI 1 (k2). From the QCAR analysis follows a possible conformation with a “hindrance” of the hydrogen atoms a and b, which occurs according to the structure in Scheme 4 when the aromatic ring rotates .
Scheme 5 demonstrates the two antipodes of TI 1 . Only the shape on the left leads to steric hindrance of the hydrogen atoms mentioned above, but not the structure shown on the right. This could be an indication of the stereospecificity of the tetrahedral intermediate TI1 (in connection with the results of the D isotope measurements - see below).
The investigations into the pH value dependency, demonstrated on the DPP 4 made from pig kidney bark, resulted in an optimum pH of approx. 6.7. The temperature optimum is around 30 ° C. The studies also showed that a positive charge at the N-terminus is necessary for the activity of the substrates . The protonation of the N-terminal primary amino group is necessary here. But it is not a requirement. As can be seen from Table 1, the existence of a positive charge is sufficient for substrate recognition. Strictly speaking, DPP 4 is not an aminopeptidase, but an onium acylaminoacyl peptidase. The catalytic cleavage of dipeptides from an oligo- or polypeptide from the N-terminal end is, from a physiological point of view, an essential process.
Secondary D isotope effects and solvent isotope effects in D 2 O
A possible model of the TI1 is shown in Scheme 4 and Scheme 5 (left). Its confirmation should be possible through D isotope effects if, on the one hand, there is an H / D exchange in the asymmetric center of the P1 amino acid and, on the other hand, an H / D exchange in the aromatic ring in the o-position. It turns out that secondary D isotope effects can be measured in both cases.
With Ala-Ala-pNA (L-Ala-L-Ala-d1-pNA) secondary D-isotope effects can be measured in the pH optimum at room temperature. In k cat = 1.27 and in K M = 1.24. If the substrate in the aromatic ring is completely deuterated (Ala-Ala-NH-C 6 D 4 -pNO 2 ), secondary D isotope effects in k cat and in K M of 1.05 ± 0.03 result.
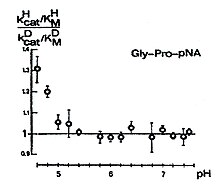
It is interesting that a secondary D isotope effect occurs in k cat / K M , according to Scheme 6, depending on the pH value. The curve is remarkable. With falling pH values from about 7.5 to about 5.0, no isotope effect can be measured. From pH 5.0 onwards, an increasing secondary D isotope effect is observed with a falling pH value, combined with a transition from k 3 to k 2 as a rate-determining step.
This result underlines the high probability of the structure shown in Scheme 4.
In the case of the substrates Ala-Pro-pNA and Gly-Pro-pNA, measurements of the solvent-D isotope effect show a one-proton transition with the rate-determining step in the deacylation and a one-proton transition for the Ala-Ala-pNA, the rate-determining step in the Acylation lying, a two-proton transition (Table 4).
| Substrate | pH | best adaptation to the function (significant according to F-test)
|
parameter | Proton transitions |
|---|---|---|---|---|
| Ala-Pro-pNA | 7.5 |
|
k cat k cat / K M |
1 1 |
| Gly-Pro-pNA | 6.8 |
|
k cat k cat / K M |
1 1 |
| Ala-Ala-pNA | 7.5 |
|
k cat k cat / K M |
(2) 2 |
Inhibitors
A number of dipeptides were examined for their effect as (competitive) inhibitors of DPP 4. Table 5 shows an overview.
| Dipeptides | K i in M |
|---|---|
| Gly-Pro | (1.6 ± 0.2) · 10 −3 |
| Val-Pro | (1.0 ± 0.1) · 10 −5 |
| Leu-pro | (7.0 ± 0.7) · 10 −5 |
| Ile-Pro | (6.9 ± 0.3) · 10 −6 |
| Asp-Pro | (2.0 ± 1.5) · 10 −4 |
| Arg-Pro | (4.2 ± 1.0) · 10 −5 |
| Phe-Pro | (4.7 ± 1.5) · 10 −5 |
| ε-Z (4-NO 2 ) Lys-Pro | (1.1 ± 0.2) · 10 −6 |
| β-Ala-Pro | (1.7 ± 0.1) · 10 −2 |
| Ala-ala | (4.1 ± 0.7) · 10 −4 |
| Ile-Ala | (1.0 ± 0.1) · 10 −5 |
| Ala-D-Ala | (1.8 ± 0.2) · 10 −3 |
| Pro-Gly | (9.0 ± 0.4) · 10 −3 |
| Gly-Phe | (3.2 ± 0.5) · 10 −3 |
| Ile-Val | (4.0 ± 0.1) · 10 −5 |
| ε-Z (4-NO 2 ) Lys-D-Pro | (4.4 ± 0.3) · 10 −5 |
| ε-Z (4-NO 2 ) Lys-Pip | (2.2 ± 0.1) · 10 −5 |
| ε-Z (4-NO 2 ) Lys-D-Pip | (2.0 ± 0.1) · 10 −4 |
| ε-N-CapronylLys-Pro | (4.8 ± 0.1) · 10 −5 |
| ε-N-Palmityl Lys-Pro | (8.1 ± 0.1) · 10 −5 |
Of these dipeptides, which are also cleavage products of DPP 4 catalysis, the Ile-Pro with a K i value of about 7.0 · 10 −6 M and the ε-Z (4-NO 2 ) Lys-Pro (K i about 10 −6 ) interesting. The corresponding pyrrolidides are about an order of magnitude more effective (Table 6).
| X-pyrrolidide X = |
pH | K i in M |
|---|---|---|
| Ile | 6.3 | (3.3 ± 0.6) · 10 −7 |
| Ile | 7.6 | (1.9 ± 0.4) · 10 −7 |
| Phe | 6.3 | (2.5 ± 0.7) · 10 −6 |
| Phe | 7.6 | (2.3 ± 0.2) · 10 −6 |
| ε-Z (4-NO 2 ) Lys | 6.3 | (3.9 ± 0.5) · 10 −7 |
X-ray crystal structure of dipeptidyl peptidase 4
Intensive studies in the field of X-ray crystal structure analysis of the DPP 4 are currently in progress. The enzyme usually consists of two identical subunits. Each of these subunits has an N-terminal peptide sequence that anchors the protein to the surface of a cell. Higher aggregates were also found in the soluble DPP 4, consisting of four identical subunits.
For certain statements, it is better to display the Conolli surface that is not so detailed. This shows that the DPP 4 has an unusual surface structure. The active center is not located on the surface outside, but inside the protein. Access to the active center is possible through a “tube” that has a larger and a smaller opening. The question is how and, above all, where is z. B. the substrate entry?
If you look at the electrostatic potential at the surface, you can see that there is a very strong negative potential near and inside the large opening with a gradient in the direction of the opening (Figure 3). In contrast, the smaller opening does not have such a strong negative potential. It is therefore conceivable that the substrates with the positively charged N-terminus, which characterizes the potential of larger areas of the recognition structure of the substrates, are “drawn into” this opening. (Representation of a subunit, human DPP 4).
The structure of the tetrahedral intermediate TI1
From the experimental finding that the D-Ala-Ala-pNA acting as substrate acts via kcat, KM remaining unaffected compared to that of the substrate Ala-Ala-pNA (see Table 3), the interpretation suggests that the N- terminal aminoacyl residue (probably with the positive charge) interacts directly with the tetrahedral intermediate in the P1 position. Therefore, a hypothetical conformation was constructed as follows (Scheme 7).
There appear to be two types of hydrogen bonds , namely those responsible for direct bonding in the active site, i.e. That is, bring the substrate or the inhibitor to the interactively active residues of the protein and those that are a direct part of the functional mechanism. If tetrahedral intermediates TI1 and TI2 (see Scheme 3) arise in the course of the functional mechanism of the serine proteases , then TI1 is asymmetric. When the carbonyl atom of the peptide bond to be cleaved is erected, two antipodal conformations can develop, one of which should recognize the peptide as a substrate (1) and the other as an inhibitor (2) (Scheme 5).
The decision in which direction the carbonyl group stands up, i.e. That is, which of the antipodal tetrahedral intermediates is preferentially formed will depend on the direction in which the interactions of the protein side chains in the active center (e.g. hydrogen bonds) are most effective or the spatial adaptation of the effector molecule is most effective. It is entirely possible that an inhibitor molecule can also be a substrate. A decisive driving force behind the separation of a part of the molecule from the tetrahedral intermediate is whether the principle of stereoelectronic control according to Delongchamps is valid. It says that the cleavage of esters or amides via a tetrahedral intermediate can only take place if both heteroatoms remaining on the central sp3 carbon atom align one of their lone electron pairs in an antiperiplanar manner to the cleaving bond.
The X-ray structure analysis of the DPP 4 / Pro-Pro-boronic acid complex confirms the structure assumed in Scheme 7 in an impressive way (Figure 4). In addition, it is clear from the hypothetical scheme 7 why the DPP 4 cannot remove any N-terminal amino acids, but only cleaves off N-terminal dipeptides. It is of interest that the boronic acid complex actually forms a tetrahedral structure, that an interaction takes place on the one hand with the Tyr547 of the protein and an O atom of the TI (distance = 2.04 A) and that on the other hand between the Asn710 of the protein and the oxygen atom of the Carbonyl group of the N-terminal peptide bond an interaction occurs (distance = 1.93 Å). The latter H-bridge is useful. It promotes the erection of the carbonyl group of the N-terminal peptide bond in the sense shown above (see Scheme 7). These relationships can be seen in the section of the X-ray crystal structure of the DPP 4 / boronic acid complex (Figure 4).
Similar images show the structures generated by Diprotin A (Figure 5 - right) and tertBuGly-Pro-Ile (Figure 5 - left). Asymmetrical tetrahedral conformations arise here. They appear to be antipodal to the structure assumed in Scheme 7. Should these real antipodal structures determined by the X-ray structure be characteristic of transition-state inhibitors? This hypothetical assumption could explain why the connections
- Diprotin A (Ile-Pro-Ile)
Diprotin B (Val-Pro-Leu) and
TertBuGly-Pro-Ile,
which basically have a substrate structure, are inhibitors. The term “hypothetical” expresses that the X-ray structures shown here (Figure 5) represent the conformations of inhibitors, the conformations of the TI (TI1) of substrates cannot be represented under the experimental conditions of X-ray crystal structure analysis.
The question: Are diprotin A or diprotin B substrates or inhibitors of DPP 4? - was published in 1991 by Rahfeld et al. posed. Umezawa et al. report that both diprotins act as inhibitors of DPP 4. Rahfeld stated that both Ile-Pro-Ile and Val-Pro-Leu are substrates of the DPP 4, as expected. The authors speak of the inhibitor effect as a “kinetic artifact”. The X-ray crystal structure studies confirm the conformation in TI1, which should be assigned to the inhibitor molecule, because the substrate structure cannot be determined with certainty under the crystallization conditions. It is all the more astonishing that the diprotins are completely hydrolyzed in an aqueous medium (kinetic measurements) after a long time (after about 20.5 hours under the conditions given in the literature). Obviously, an equilibrium of the antipodal TI conformations gradually re-establishes itself in dilute aqueous solution. The dualism of the diprotins becomes clear through such a mechanism. Their K i and K M values are shown in Table 7.
| parameter | Val-Pro-Leu | Ile-Pro-Ile |
|---|---|---|
| k cat in s −1 | 27.00 ± 0.05 | 1.29 ± 0.02 |
| K M in M | (1.63 ± 0.10) · 10 −5 | (3.50 ± 0.30) · 10 −6 |
| k cat / K M in M −1 · s −1 | (1.66 ± 0.10) · 10 6 | (3.69 ± 0.30) · 10 5 |
| K i in M | (1.88 ± 0.40) · 10 −5 | (1.28 ± 0.36) · 10 −6 |
Natural substrates of dipeptidyl peptidase 4
(see dipeptidyl peptidase 4 )
Final remarks
Dipeptidyl peptidase 4 is of great interest physiologically and pharmacologically . Some active and specific inhibitors of the enzyme have been identified as potential agents against type II diabetes mellitus . The two dipeptidyl peptidase-4 inhibitors sitagliptin and vildagliptin have now been approved as drugs for the treatment of type 2 diabetes mellitus . There is evidence that DPP 4 or related enzymes play a role in wound healing processes , possibly in a number of cancers (according to English-language publications, dipeptidyl peptidase IV and aminopeptidase N also appear to be involved in the development of thyroid carcinomas) and in the pathogenesis of AIDS play.
The close relationship between the enzymes DP II and PSE (proline-specific endopeptidase), also known as PPCE ( Post Proline Cleaving Enzyme ), to DPP 4 was described a few years ago . More recently it has been found that DPP 4 is a member of a family of dipeptidyl peptidases. It can be assumed that comparative mechanistic and physiological studies in this area will result in further insights into theoretical and applied questions.
Individual evidence
- ↑ Hopsu-Havu VK, Glenner GG: A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide . In: Histochemistry . 7, No. 3, 1966, pp. 197-201. PMID 5959122 .
- ↑ Horst Schulz, "Contributions to the purification and characterization of a dipeptidylaminopeptidase from pig kidneys" Dissertation at the University of Halle, submitted at the end of 1972, defended in March 1973
- ^ Alfred Barth, Horst Schulz, Pharmazie 29, 195, 1974
- ^ Alfred Barth, Horst Schulz, Klaus Neubert, Acta biol. med. germ. 37, 157, 1974
- ^ Printer DJ: Therapeutic potential of dipeptidyl peptidase IV inhibitors for the treatment of type 2 diabetes . In: Expert Opin Investig Drugs . 12, No. 1, January 2003, pp. 87-100. doi : 10.1517 / 13543784.12.1.87 . PMID 12517256 .
- ↑ Entry in the Enzyme Nomenclature of the NC-IUBMB
- ↑ Heins J, Welker P, Schönlein C, et al. : Mechanism of proline-specific proteinases: (I) Substrate specificity of dipeptidyl peptidase IV from pig kidney and proline-specific endopeptidase from Flavobacterium meningosepticum . In: Biochim. Biophys. Acta . 954, No. 2, May 1988, pp. 161-9. PMID 2896517 .
- ↑ Rahfeld J, Schutkowski M, Faust J, Neubert K, Barth A, Heins J: Extended investigation of the substrate specificity of dipeptidyl peptidase IV from pig kidney . In: Biol. Chem. Hoppe-Seyler . 372, No. 5, May 1991, pp. 313-8. PMID 1678608 .
- ↑ Barth, A.: Four decades in the service of science. In: "Acta Facult.Pharm.Univ.Comenianae", 52,236-250,2005
- ↑ Schutkowski, M.: "Dissertation at the University of Halle
- ↑ See Küllerts, G., Fischer, G., Barth, A .: Contributions to the catalysis mechanism of Dipeptidyl Peptidase IV "Acta biol. Med. Germ.", 37, 559-567, 1978
- ↑ Küllertz, G., Oehme, P., Barth, A., (Ed.) ,: Dipeptidyl Peptidase IV - Chemistry, Biochemistry and Physiological Aspects, "Contribution Act.Forsch., 1981. Also as: Küllertz, G ,. Oehme, P., Barth, A. In: "Pharmazie", 36, No. 7.518 ff, 1981
- ↑ Brandt, W., Lehmann, T., Hofmann, T., Schowen, RL., Barth, A .: The probable conformation of substrates recognized by dipeptidyl-peptidase IV and some aspects of the catalytic mechanism derived from theoretical investigations , " J. Comput. Aided Mol. Des. ", 6, No.2, 159-174, 1992
- ↑ P. Delongchamps, Stereo electronic control in the cleavage of tetrahedral intermediates in the hydrolyses of esters and amides, "Tetrahedron" 31, 2463-2490, 1975
- ↑ a b Engel M, Hoffmann T, Manhart S, et al. : Rigidity and flexibility of dipeptidyl peptidase IV: crystal structures of and docking experiments with DPIV . In: J. Mol. Biol. . 355, No. 4, January 2006, pp. 768-83. doi : 10.1016 / j.jmb.2005.11.014 . PMID 16330047 .
- ↑ Barth A., Thondorf I., Kovacs P. "Acta Facult.Pharm.Univ.Comenianae", 55.5.2008
literature
- A. Barth: The chemistry of dipeptidyl peptidase IV - an overview. In: Digital Library Saxony-Anhalt. MLU Halle-Wittenberg, Faculty of Mathematics, Natural Sciences and Technology, April 22, 2010, accessed on April 19, 2011 .
- A. Barth, I. Thondorf, J. Stano: Thoughts on the mechanism of serine proteases, Part I - The function of the tetrad: Asp… .His… .Ser… .Xaa. In: Acta Facult. Pharm. Univ. Comenianae 51: 15-26 (2004).
- A. Barth, I. Thondorf, S. Gebauer, W. Brandt, K. Neubert, J. Stano, M. Psenak, P.Kovacs: Thoughts on the Mechanism of Serine Proteases , Part II – Dipeptidyl-Peptidase IV (DP IV / CD 26). In: Acta Facult. Pharm. Univ. Comenianae 52, 7-21 (2005).
- A. Barth, I. Thondorf, S. Gebauer, K. Neubert, P. Kovacs, J. Stano: Thoughts on the Mechanism of Serine Proteases, Part III - a) The tetrahedral intermediate in the acylation process. b) The boronic acids . In: Acta Facult. Pharm. Univ. Comemianae 53, 5-15 (2006)
- A. Barth, I. Thondorf, K. Neubert, S. Hoffmann, P. Kovacs, J. Stano: Thoughts on the Mechanism of Serine Proteases, Part IV - Transition State Inhibitors - The Asymmetrical Tetrahedral Intermediate TI 1. In: Acta Facult. Pharm. Univ. Comenianae 53: 16-21 (2006).
- A. Barth, I. Thondorf: "Thoughts on the Mechanism of Serine Proteases, Part V - Dipeptidyl Peptidase IV -Biochemical-Physiological Aspects" In: "Acta Facult." Pharm. Univ. Comenianae 54: 7-13 (2007).
- A. Barth, I. Thondorf, P. Kovacs: "Thoughts on the mechanism of serine proteases, Part VI - DP IV inhibitors. Active ingredients against type II diabetes with a novel mechanism of action" In: "Acta Facult." Pharm. Univ. Comenianae 55, 5-10 (2008)
- A. Barth: "Thoughts on the Mechanism of Serine Proteases, Part VII - An Overview [1966 - 2006]" In: "Acta Facult." Pharm. Univ. Comenianae 55, 11-22 (2008)
- S. Ansorge (Ed.): Abstracts of the 2nd International Conference on Dipeptidyl Aminopeptidases - Basic Sciences and Clinical Applications . Magdeburg, Germany 2005.
- U. Langdeckel, D. Reinhold, U. Bank (Eds.): Dipeptidyl Aminopeptidases - Basic Science and Clinical Applications (Proceedings) in Advances in Experimental Medicine and Biology. Vol. 575, Springer 2006.
- W. Brandt, T. Hofmann: The Recognition Conformation of Substances of the Dipeptidyl Peptidase IV . In: Biol. Zentr. Bl. 107: 21-30 (1978).
- G. Küllertz, G. Fischer, A. Barth: Contributions to the catalysis mechanism of dipeptidyl peptidase IV . In: Acta biol. med. germ. 37: 559-567 (1978).
- K. Ludwig, Y. Shuling, H. Fan, W. Reutter, Ch. Böttcher: The 3D structure of DPP IV / CD 26 as obtained by cryo-TEM and single partial analysis . In: Biochem. Biophys. Res. Commun. 304: 73-77 (2003).
- M. Engel, T. Hofmann, S. Manhart, U. Heiser, S. Chambre, R: Huber, H.-U. Demuth, W. Bode: Rigitity and Flexibility of Dipeptidyl Peptidase IV: Crystal Structures of and Docking Experiments with DP IV . In: J. Mol. Biol. 355 768-783 (2006).
- R. Thoma, B. Löffler, M. Stihle, M. Huber, W. Ruff, A. and M. Hennig: Structural basis of proline-specific exopeptidase activity as observed in Human dipeptidyl peptidase-IV . In: Structure (Camb) . 11: 947-959 (2003).
- J. Rahfeld, M. Schierhorn, B. Hartrodt, K. Neubert, J. Heins: Are diprotin A (Ile-Pro-Ile) and diprotin B (Val-Pro-Leu) inhibitors or substrates of dipeptidyl peptidase IV? In: Biochim. Biophys. Acta . 1976: 314-316 (1991).
- H. Umezawa, T. Aoyagi, H. Naganawa, M. Hamada T. Takeuchi: Diprotins A and B, Inhibitors of Dipeptidyl Aminopeptidase IV, Produced by Bacteria . In: J. Antibiotics. 37: 422-425 (1984).






![{\ displaystyle [a] / [c]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/758aec59df6790b870ca97a9246e5fac959a77ca)
![{\ displaystyle [a] / [b]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0555e5fabf1de653a28544a7111f59266419a940)
![{\ displaystyle [c] / [b]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2104156e42c96df2cb5c6d4149a2dca875de7cd1)
![[a]](https://wikimedia.org/api/rest_v1/media/math/render/svg/ea82bc70a8e322f13a3c4e5b9d5d69e8ef097ad8)
![{\ displaystyle [b]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e016d81a1b29bb79b275e446042b925b7dd6b6d7)
![[c]](https://wikimedia.org/api/rest_v1/media/math/render/svg/5a864aec9ea68d53f65cd151cbb0d662c5e5ddc1)