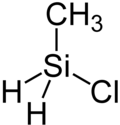
Chlorine (methyl) silane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chlorine (methyl) silane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CH 5 ClSi | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 80.59 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
0.94 g cm −3 (−80 ° C) |
|||||||||||||||
| Melting point |
−135 - −134 ° C |
|||||||||||||||
| boiling point |
−46 ° C (at 13 h Pa ) |
|||||||||||||||
| Vapor pressure |
724 hPa at 0 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chloro (methyl) silane is a chemical compound from the class of the silanes . It consists of a central silicon atom around which two hydrogen atoms and one chlorine atom and one methyl radical are coordinated in a tetrahedral manner .
presentation
Chlorine (methyl) silane can be produced by the chlorination of methylsilane with hydrogen chloride in the presence of aluminum chloride as a catalyst at an elevated temperature. Dichloro (methyl) silane is formed as a by-product .
properties
It is a compound that is gaseous at room temperature, liquefies at −46 ° C (at 13 hPa) and solidifies at −135 to −134 ° C.
use
Chlorine (methyl) silane can be used to produce organic silanes. The chlorine substituent serves as a leaving group and can be exchanged for nucleophiles in a substitution reaction . Grignard compounds or organolithium compounds , for example, can be used as nucleophiles .
swell
- ↑ a b c d A. Stock, C. Somieski: Siliciumwasserstoffe VI .: Chlorination and methylation of monosilane , in: Chem. Ber. 1919 , 52 , 710; doi : 10.1002 / cber.19190520410 .
- ↑ E. Hengge, W. Kalchauer: New ways to polysilanes , in: monthly. Chem. 1990 , 121 , 793-802.
- ↑ a b Entry on methylchlorosilane in the GESTIS substance database of the IFA , accessed on January 6, 2016 (JavaScript required)
- ↑ E. Lukevics, R. Sturkovich, Y. Goldberg, A. Gaukhman: Reactions of heteroarylhydrosilanes with dichlorocarbene under phase-transfer conditions , in: J. Organomet. Chem. 1988 , 345 , 19-25.
- ↑ E. Lukevics, V. Ryabova, P. Arsenyan, S. Belyakov, J. Popelis, O. Pudova: Bithienylsilanes: unexpected structure and reactivity , in: J. Organomet. Chem. 2000 , 610 , 8-15.



![{\ displaystyle \ mathrm {H_ {3} SiCH_ {3} + \ HCl {\ xrightarrow [{AlCl_ {3}}] {100 \, {} ^ {\ circ} C, 24h}} \ H_ {2} ClSiCH_ {3} \ + \ H_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e2f75898ed7627dbd39dd44eb514f489aa8fc519)