Chlorotrifluoromethane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
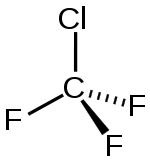
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Chlorotrifluoromethane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | CClF 3 | |||||||||||||||
| Brief description |
colorless, odorless gas with a sweet smell in higher concentrations |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 104.46 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
−189 ° C |
|||||||||||||||
| boiling point |
−81.4 ° C |
|||||||||||||||
| Vapor pressure |
3.18 M Pa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Global warming potential |
15451 (based on 100 years) |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−706.3 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Chlorotrifluoromethane is a chemical compound from the group of aliphatic , saturated halogenated hydrocarbons ( CFC ).
Extraction and presentation
Chlorotrifluoromethane can be obtained by reacting methane with chlorine and hydrogen fluoride with aluminum chloride as a catalyst.
properties
Chlorotrifluoromethane is a colorless, odorless gas that in higher concentrations has a sweet smell, similar to carbon tetrachloride . The compound decomposes above 150 ° C. It has a critical temperature of 28.78 ° C, a critical pressure of 38.6 bar, a critical density of 0.581 kg / l and a triple point temperature of −189 ° C.
use
Chlorotrifluoromethane was previously used as a refrigerant under the name R 13 or Freon 13 . Due to its global warming potential of 14,000 and its ozone depletion potential of 1, further production was prohibited in the future in the Montreal Protocol .
Individual evidence
- ↑ a b c d e f g h i j Entry on chlorotrifluoromethane in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ^ William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, 2016, ISBN 978-1-4398-8050-0 , pp. 120 ( limited preview in Google Book search).
- ↑ Honeywell: R 13 (PDF; 26 kB)
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 75-72-9 or chlorotrifluoromethane ), accessed on November 2, 2015.
- ↑ G. Myhre, D. Shindell et al .: Climate Change 2013: The Physical Science Basis . Working Group I contribution to the IPCC Fifth Assessment Report. Ed .: Intergovernmental Panel on Climate Change . 2013, Chapter 8: Anthropogenic and Natural Radiative Forcing, pp. 24-39; Table 8.SM.16 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-19.
- ↑ cfc.com: Chlorotrifluoromethane .

