Ozone hole


halogenated hydrocarbons , weighted according to
their ozone depletion potential .
An ozone hole is a strong thinning of the ozone layer , as it was first observed in 1985 at the South Pole over the Antarctic , and at the beginning of 2020, according to a report by the Alfred Wegener Institute, for the first time over the Arctic ( North Pole ). The causes of ozone depletion are mainly free radical chlorine atoms from chlorinated organic compounds , which are collectively referred to as chlorofluorocarbons (CFCs or CFCs). In addition, are Halon and teilbromierte and Partly chlorinated hydrocarbons (H-FBKW, HCFCs), bromochloromethane , carbon tetrachloride , methyl bromide and trichloroethane involved in the destruction. The thinned ozone layer lets more of the UV-B part of solar radiation through to the ground: Ultraviolet radiation can have a carcinogenic effect on living beings .
Observation history
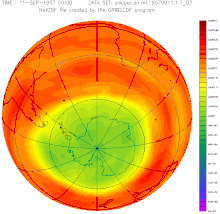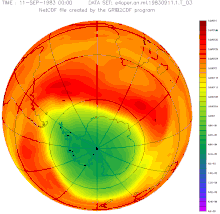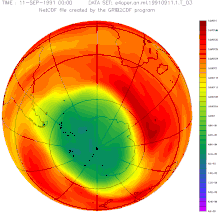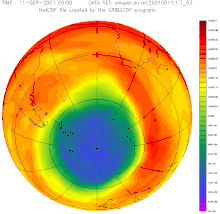
The first indications of a weakening of the ozone layer over the Antarctic existed as early as 1957, but the measurements from the British research station Halley Bay were largely ignored.
In 1974, the physical chemists Mario J. Molina and Frank Sherwood Rowland warned that the build-up of poorly degradable CFCs in the atmosphere would lead to a significant decrease in ozone concentrations, worldwide and all year round - no one had foreseen the ozone hole until then. In 1995, the two researchers, together with the atmospheric chemist Paul Crutzen, received the Nobel Prize in Chemistry for elucidating the mechanisms that contribute to the build-up and depletion of the ozone layer.
The ozone hole has occurred annually since the early 1980s: within a few weeks after sunrise in Antarctica, the ozone concentration collapses and recovers within a few months. The cause of this dynamic is the reaction of pollutants that are stored on the ice crystals of stratospheric clouds and that evaporate with the ozone after the long, cold polar night . This is broken down.
The size of the break-in grew from a few percent to more than fifty percent within a few years. The entire polar vortex is affected , an area of several million square kilometers, as the remote sensing that began at that time impressively illustrated. The dramatic development and the unequivocal scientific evidence of the causes quickly led to a worldwide ban on CFCs. In 1987, the US President Ronald Reagan and the British Prime Minister Margaret Thatcher supported the convening of an international conference at which the phasing out of several industrial chemicals was negotiated and approved in the Montreal Protocol .
The photolysis of the CFC molecules by UV-C not only releases the harmful halogen atoms, but also initiates the complete breakdown of the CFC molecule. However , as a result of this process , the lifespan of most CFC compounds is many decades, since the sun's UV-C radiation does not reach most of the atmosphere. Therefore, the hole in the ozone layer is unlikely to close until the second half of the 21st century.
After evaluating the measurements from 2012, there was talk of a reversal of the ozone trend for the first time at the South Pole: According to the management of the meteorological observatory at the Neumayer III research station , the main cause of this trend reversal is the success of the global ban on CFCs.
In September 2014, the World Meteorological Organization (WMO) published a report: The ozone hole will no longer be an issue by 2050 at the latest if the trend that research has been observing for years continues.
In autumn 2015, the German Aerospace Center (DLR) announced that, according to the latest data, the ozone hole over the Antarctic (South Pole) was around 2.5 million square kilometers larger than in the previous year and reached around 26 million square kilometers (more than the area of North America ) the largest expansion since the record year 2006 (approx. 27 million km²).
In June 2016, researchers announced that the ozone layer was actually recovering, as had been suspected for years. According to their measurements, the hole narrowed by around 4 million km² in September compared to the turning point at the turn of the millennium. According to computer simulations, at most half of this is due to weather fluctuations. The record in size from the previous year is largely due to the eruption of the Calbuco volcano .
In 2017, according to NASA , the Antarctic ozone hole reached its smallest size since 1988, at around 20 million square kilometers. While the peak always had an ozone concentration of 0 at any altitude in the stratosphere, this was not the case this year, according to the weather balloons . NASA named natural fluctuations due to unusually warm stratospheric temperatures over the Antarctic as the cause of this small expansion. This reduced the formation of clouds over the Antarctic, which promotes the thinning of the ozone layer. These clouds in turn are an important starting point for chemical reactions in which the ozone is destroyed with the help of the pollutants chlorine and bromine, which serve as catalysts. The less pronounced ozone hole, however, is not a signal for a hoped-for faster healing. Scientists expect UV protection to largely recover by around 2070; the concentration of ozone-depleting substances in the atmosphere is currently still too high.
While the ozone concentration in the upper stratosphere has increased again since 1998, the ozone concentration in the lower stratosphere continues to decrease between the 60th parallel north and the 60th parallel south. Most of the ozone layer is in the lower stratosphere. Overall, the ozone layer has therefore become thinner. The causes of this phenomenon have not yet been researched.
Research history of ozone depletion
Walter Noel Hartley concluded in 1881 from a comparison of the absorption spectrum of ozone in the UV-B range (Hartley band) with the steeply falling solar spectrum there must be more ozone in the upper atmosphere than could be chemically detected on the ground . Quantitative measurements became routine around 1930 using the Dobsonian spectrophotometer . At the same time, Sydney Chapman developed the first reaction mechanism that explained the relative stability of the ozone layer, the ozone-oxygen cycle .
According to this, the ozone in the stratosphere is both formed and split by ultraviolet radiation from the sun. The split off oxygen atom usually combines again with an oxygen molecule to form ozone, with a loss occurring through occasional reactions between oxygen atoms and ozone, which limits the increase in the ozone concentration.
This model correctly predicted that in the altitude range of UV-C absorption (30–60 km) the ozone concentration decreases with the air density, which was confirmed with sounding rockets in the early 1970s. The reason is that the reaction of the oxygen atom, which leads back to ozone,
- O + O 2 → O 3 ,
is actually a trimolecular reaction: A third collision partner M is required, which dissipates the binding energy as kinetic energy:
- O + O 2 + M → O 3 + M (M = N 2 or O 2 )
The smaller (with increasing altitude) the concentration of M, the longer the oxygen atom is exposed to loss reactions.
As early as 1930, however, the flaw of this model became obvious: It is based on an ozone layer that should be about three times as thick as it actually is. Therefore, further loss processes were looked for. At first one suspected HO x ( HO , HO 2 ), whereby the H atom comes from compounds that are broken down sufficiently slowly in the troposphere that they reach the stratosphere: H 2 , H 2 O and CH 4 . In the lower stratosphere, where UV-C is insufficient, they are split up by O (1D), e.g. B.
- H 2 O + O (1D) → 2 HO •.
O (1D) is the electrically excited oxygen atom that is created during the photolysis of ozone,
- O 3 + UV-B → O 2 + O (1D),
which runs the risk of being de-excited to an O atom in the electronic ground state with every impact, e.g. B.
- O (1D) + O 2 → O (3P) + O 2 .
The excitation energy is converted into kinetic energy of the reaction products, i.e. into heat.
In the upper stratosphere these compounds are split by UV-C, e.g. B.
- CH 4 + UV-C → H • + • CH 3
and then in quick succession
- • CH 3 + O 2 → CH 3 OO •
- CH 3 OO • + O 3 → CH 3 O • + 2 O 2
- CH 3 O • + O 2 → HCHO + HOO •
- HCHO + UV-A → H • + HCO • (alternatively → H 2 + CO)
- HCO • + O 2 → HOO • + CO
- H • + O 2 → HOO •
The resulting hydroperoxyl radicals, HOO •, henceforth intercept O atoms that would otherwise form ozone:
- HOO • + O → HO • + O 2 ,
without being used up, because HO • forms again HOO • with further ozone depletion:
- HO • + O 3 → HOO • + O 2 .
This catalytic cycle explains a good part of the lower observed ozone column heights.
Another substance that can get into the stratosphere and form radicals there is laughing gas , N 2 O. This is also photolyzed by UV-C or reacts with O (1D). The nitrogen oxides NO and NO 2 are formed , which are in equilibrium with each other:
- NO + O 3 → NO 2 + O 2
- NO 2 + visible light → NO + O.
This pair of reactions represents an accelerated version of ozone photolysis, because the visible light of the sun is much more intense than the UV-B light, which is necessary for the photolysis of ozone. Nitrogen oxides contribute indirectly to ozone depletion, since the O atoms do not completely reform ozone (see above).
In 1970 Crutzen warned against the introduction of nitrogen oxides by supersonic aircraft like the Concorde (planned in large numbers at the time), which fly in the lower stratosphere.
The chemical process of ozone depletion
Chlorine atoms and other radicals X • lead to additional losses, usually according to the following catalytic cycle:
- X • + O 3 → XO • + O 2
- XO • + O → X • + O 2
The second reaction determines the rate, because O atoms are comparatively scarce even during the day. Therefore, z. B. Chlorine in the stratosphere predominantly not as Cl •, but as ClO •. Especially at twilight, the (possibly different) radicals XO • react with one another, e.g. B.
- ClO • + Br O • → Cl • + Br • + O 2
In this case, too, the two radicals X • are released again. For some other combinations of XO • this is not the case, important examples are
- ClO • + ClO • → Cl 2 + O 2 and
- ClO • + NO 2 • → ClONO 2
The resulting reaction products are called reservoir species, because the radicals are only temporarily bound in them, mainly at night, while they are more or less rapidly photolyzed during the day. During the long twilight of the polar night , the longer-lived chlorine nitrate ClONO 2 is created , because UV-C is necessary to split it, while for chlorine Cl 2 the much more intense UV-A is sufficient when the sun is low.
The nocturnal formation of polar stratospheric clouds (PSC, polar stratospheric clouds ) is responsible for the pronounced ozone hole, especially in spring . Usually there are no clouds in the stratosphere because it is too dry. At the particularly low temperatures of the polar night, particularly the southern one, down to below −80 ° C, however, residues of water vapor H 2 O can freeze out together with nitric acid HNO 3 . Nitric acid is created from nitrates, such as the above chlorine nitrate:
- ClONO 2 + H 2 O → HClO + HNO 3 ,
Halogen compounds, such as the hypochlorous acid HClO here, remain in the gas phase and are quickly split at sunrise. Suddenly there are a lot of ozone-depleting radicals. The PSC only gradually evaporate and bring the nitrogen compounds back into the air, which form reservoir species with the chlorine radicals and thus dampen the breakdown of ozone. According to research results from NASA, the ozone depletion by CFCs can only be insufficiently explained by the release of chlorine radicals.
Appearance at the south and north poles
The regular strong expression of the Antarctic polar vortex is the main cause of the very low temperatures in its center, down to below 190 K (approx. −80 ° C).
The Arctic polar vortex, on the other hand, is usually not cold enough for stratospheric clouds, so that there has not yet been a clear ozone hole; The ozone hole that was detected by the Alfred Wegener Institute over the Arctic on 20 million square kilometers for two weeks in March 2020 is due to a particularly strong polar vortex and low temperatures in the stratosphere there this season.
The four adjacent figures show the northern hemisphere at the top, the southern hemisphere at the bottom, on the left the color-coded annual mean of the ozone column height for 1979 (before the discovery of the ozone hole), on the right for 2007.
Natural halogen compounds
Sea salt (NaCl) is water soluble and is washed out of the atmosphere before it reaches the stratosphere.
Plants, on the other hand, make a measurable contribution to ozone-depleting compounds. Cruciferous plants produce methyl bromide , which is about 80 times more harmful than CFCs. Rapeseed alone produces 6,600 tons a year, 15 percent of the amount that is still produced industrially. In addition, methyl bromide, which was formerly also used by farmers for pest control in fields under tarpaulin, is also used to fumigate international fruit transports and furniture transports against pests. In contrast, evergreen trees and potatoes synthesize methyl chloride .
Halogen compounds also escape during volcanic eruptions : While hydrogen chloride is largely washed out like sea salt, bromine compounds can impair the ozone layer, at least locally. When a super volcano erupts , the ozone layer is severely damaged. The last one took place 74,000 years ago.
Based on the research results of the environmental physicists at Heidelberg University, scientists suspect that the ozone layer may be damaged by natural chlorine, bromine and possibly iodine compounds, which are mainly formed in the coastal areas of the oceans by aquatic plants and microorganisms. A research project is to investigate the natural sources of halogenated hydrocarbons and the atmospheric transport and degradation of these ozone-depleting compounds. In the meantime, studies in coastal waters and in the atmosphere above the waters as part of a research project have confirmed these assumptions.
Nitrous oxide ("laughing gas") has now replaced chlorofluorocarbons (CFCs) as the most important source of ozone-damaging emissions of the 21st century .
literature
- Joe C. Farman et al .: Large losses of total ozone in Antarctica reveal seasonal ClOx / NOx interaction . In: Nature , Volume 315, 1985, pp. 207-210, doi: 10.1038 / 315207a0 .
- Martin Dameris, Thomas Peter, Ulrich Schmidt, Reinhard Zellner : The ozone hole and its causes , in: Chemistry in our time 2007 , 41 , 152–168; doi: 10.1002 / ciuz.200700418 .
- MZ Jacobson: Atmospheric Pollution: History, Science and Regulation , Cambridge Univ. Press, 2012, ISBN 978-1-107-02161-7 , chap. 11: Global Stratospheric Ozone Reduction ( ppt presentation ).
- Marcel Nicolet: Stratospheric Ozone: An Introduction to its Study , Reviews of Geophysics and Space Physics, Vol. 13, 1975, doi: 10.1029 / RG013i005p00593 - a summary of the state of knowledge about the ozone layer at the time of the CFC warning by Molina and Rowland.
- Reiner Grundmann : Transnational environmental policy to protect the ozone layer. USA and Germany in comparison . Campus Verlag, Frankfurt a. M. 1999, ISBN 3-593-36222-8 .
- World Meteorological Organization: Scientific assessment of ozone depletion 2002 , Global Ozone Research and Monitoring Project - Report No. 47, WMO, Geneva 2003, ISBN 92-807-2261-1 .
- NASA Panel for Data Evaluation: Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies , Evaluation No. 14, JPL Publication 02-25, 2003, online (PDF; 3.2 MB).
- Konrad Mauersberger: The ozone hole over the South Pole . In: The Geosciences. Volume 9, Issue 11, 1991, doi: 10.2312 / geoswissenschaften . 1991.9.352 . Pp. 352-356.
- Wolf-Andreas Liebert: Knowledge transformations: action semantic analyzes of science and communication texts. Walter de Gruyter, Berlin 2002, ISBN 978-3-11-017276-8 , online excerpt - The book reproduces the ozone hole debate of the 1980s and analyzes the change that knowledge experiences when it is transferred from the scientific to the popular scientific area.
- Greenpeace: The Ozone Layer and the Ozone Hole , 1996.
See also
Web links
- Center for Atmospheric Science, Cambridge University, UK: Ozone Hole - History and Science
- Data Archive NASA the ozone hole (English)
- Daily updated maps of the ozone distribution in the northern hemisphere , from the "Ozone Mapping Center" of the World Meteorological Organization (English)
- Press release on the award of the Nobel Prize to Mario J. Molina, Frank Sherwood Rowland and Paul J. Crutzen
Individual evidence
- ↑ Ross J. Salawitch, David W. Fahey, Michaela I. Hegglin, Laura A. McBride, Walter R. Tribett, Sarah J. Doherty: Twenty questions and answers about the ozone layer, 2018 update: Scientific assessment of ozone depletion, 2018 . Ed .: World Meteorological Organization. Geneva, Switzerland 2019, ISBN 978-1-73293-172-5 , pp. 9 (English, noaa.gov ).
- ↑ a b North Pole - Alfred Wegener Institute reports of a large ozone hole over the Arctic. Retrieved on March 28, 2020 (German).
- ↑ Bernhard Pötter: At the last minute . Article dated September 6, 2007 in the zeit.de portal (accessed April 17, 2018)
- ↑ Mario J. Molina and FS Rowland : Stratospheric sink for chlorofluoromethanes: chlorine atom-catalysed destruction of ozone. In: Nature . Volume 249, No. 5460, 1974, pp. 810-812, doi: 10.1038 / 249810a0 .
- ↑ Spectrum of Science : Mechanisms of Ozone Depletion in the Stratosphere , December 1995 (monthly spectrum ).
- ↑ Interview with Nobel Prize winner Paul Crutzen , conducted by Astrid Gräslund in June 2000 (from 4:30 p.m.).
- ↑ Richard L. McKenzie et al .: Ozone Depletion and Climate Change: Impacts on UV Radiation , Photochem Photobiol Sci, 2011, pp. 182-198, doi: 10.1039 / c0pp90034f : In middle to high latitudes, UV-B is still towards the end of the century will be 5 to 20% lower than in 1980.
- ↑ sueddeutsche.de , June 13, 2013: The ozone hole closes .
- ↑ Page no longer available , search in web archives: Hessischer Rundfunk: Darling, we have shrunk the ozone hole, From the end of a threat .
- ↑ Badische-zeitung.de , October 24, 2015: The ozone hole is growing again (October 26, 2015).
- ^ Zeit.de , October 26, 2015, Interview Dagny Lüdemann with Michael Bittner (DLR): "Such a gigantic ozone hole surprised us" (October 26, 2015).
- ↑ Decisive evidence: the ozone hole closes. In: spiegel.de , June 30, 2016.
- ↑ Susan Solomon, Diane J. Ivy, Doug Kinnison, Michael J. Mills, Ryan R. Neely: Emergence of healing in the Antarctic ozone layer . In: Science . tape 353 , no. 6296 , July 15, 2016, p. 269-274 .
- ↑ NASA: Ozone hole smallest it's been since 1988 . In: CNET . ( cnet.com [accessed November 3, 2017]).
- ↑ Sara Blumberg: Warm Air Helped Make 2017 Ozone Hole Smallest Since 1988 . In: NASA . November 2, 2017 ( nasa.gov [accessed November 3, 2017]).
- ↑ Ozone hole as small as it has not been for decades . In: Spectrum of Science , November 4, 2017. Accessed November 4, 2017.
- ^ William T. Ball et al .: Evidence for a continuous decline in lower stratospheric ozone offsetting ozone layer recovery . In: Atmospheric Chemistry and Physics . tape 18 , 2018, p. 1379-1394 , doi : 10.5194 / acp-18-1379-2018 .
- ^ Francis D. Pope, Jaron C. Hansen, Kyle D. Bayes, Randall R. Friedl, Stanley P. Sander: Ultraviolet Absorption Spectrum of Chlorine Peroxide, ClOOCl. In: The Journal of Physical Chemistry A. 111, 2007, p. 4322, doi : 10.1021 / jp067660w .
- ↑ Quirin Schiermeier: Chemists poke holes in ozone theory. September 26, 2007, accessed December 15, 2018 .
- ↑ https://www.deutschlandfunk.de/ausstieg-aus-dem-methylbromid.697.de.html?dram:article_id=71795
- ↑ Spectrum of Science : Environmental Toxins from Nature's Gift Table , June 2005.
- ↑ Spectrum of Science : The Elemental Force of the Supervolcanoes , August 2006.
- ↑ Do halogen compounds from natural sources damage the ozone layer? IDW-Online November 4, 2011 .
- ↑ Coastal waters produce ozone-depleting halogen compounds IDW-Online February 1, 2012 .
- ^ AR Ravishankara et al .: Nitrous Oxide (N 2 O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century . In: Science . Epub, 2009. PMID 19713491 .