Chrysoidine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
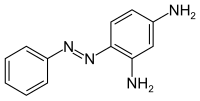
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Chrysoidine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 12 H 12 N 4 | ||||||||||||||||||
| Brief description |
yellow needles |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 212.25 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
117.5 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Chrysoidin or 2,4-diaminoazobenzene is an azo dye that was discovered by Heinrich Caro in 1875 and specifically synthesized by Otto Nikolaus Witt in 1876 . The dye with the color index name Solvent Orange 3 belongs to the application group of solvent dyes . The hydrochloride of chrysoidin, on the other hand, is the cationic dye CI Basic Orange 2.
synthesis
Chrysoidin can be prepared by an azo coupling of diazotized aniline with 1,3-diaminobenzene .
use
Chrysoidin is approved for coloring tannin-stained cotton, leather and paper as well as in histology for coloring Corynebacterium diphtheriae . On the other hand, use in cosmetic products is prohibited. The dye is used in FCA staining in botany.
Chrysoidin dyes wool, silk and stained cotton golden yellow, pulling a little orange.
Individual evidence
- ↑ a b c d e f g Entry on 2,4-diaminoazobenzene. In: Römpp Online . Georg Thieme Verlag, accessed on July 6, 2014.
- ↑ a b c d Entry on chrysoidin in the GESTIS substance database of the IFA , accessed on February 8, 2018(JavaScript required) .
- ↑ External identifiers or database links for chrysoidin hydrochloride (CI Basic Orange 2) : CAS number: 532-82-1, EC number: 208-545-8, ECHA -InfoCard: 100.007.770 , GESTIS- Substance database : 104818 , PubChem : 10771 , ChemSpider : 10468547 , Wikidata : Q17522036 .
- ↑ Entry on 4- (phenylazo) benzene-1,3-diamine in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Otto Nikolaus Witt: The Chrysoïdin and its implementations . In: Reports of the German Chemical Society . tape 10 , no. 1 , January 1877, p. 654–662 , doi : 10.1002 / cber.187701001183 ( PDF ).
- ↑ Fuchsin-Chrysoidin-Astrablau according to Etzold (FCA staining)
- ↑ Adolf Beythien, Ernst Dressler (Ed.): Merck's Lexicon of Goods for Trade, Industry and Commerce . 7th edition. Leipzig 1920, ISBN 3-933497-13-2 , entry on chrysoidin (reprint: Manuscriptum, Recklinghausen 1996): “(Diamidoazobenzene hydrochloride, diamidoazobenzene hydrochloric acid), a yellow tar dye belonging to the group of azo dyes, is produced by the action of metaphenylenediamine on hydrochloric acid diazobenzene . It dissolves in water and dyes wool and silk, as well as cotton stained with tannin, golden yellow, turning a little orange. The hot, concentrated, aqueous solution turns into a blood-red gel when it cools. "


