Cluster (physics)
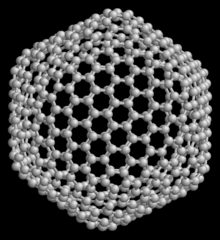
C 540 is a very unstable spherical structure due to its enormous size, because it is only spherical . Such large fullerenes are therefore onlystablein connection with C 60 , C 70 and larger fullerenes as so-called "onions".
A cluster is a collection of atoms or molecules with a number of atoms between 3 and 10 million.
Due to their small size, clusters have properties that differ from those of a macroscopic solid . Concepts from atomic and molecular physics often fail when it comes to explaining the properties of such small particles. The properties of the clusters thus represent a link between atomic and molecular physics on the one hand, and solid-state physics on the other. The subject of research in this area is how the macroscopic properties of a solid develop from the properties of an atom or molecule. The ratio of surface to volume atoms is decisive for many properties of the cluster.
A further distinction is made between a free cluster and a deposited cluster. The latter is on a surface, while the free cluster moves freely in space.
Classification
In general, the clusters are divided according to the number of atoms ( n ):
- n = 3–12 atoms ( microclusters ): For microclusters, all components of the cluster are still on the surface . Concepts and methods of atomic and molecular physics and surface physics are applicable and useful.
- n = 13–100 atoms ( small clusters ): The properties of small clusters are largely determined by quantum effects . The electronic energy levels are already quite close to one another, but the number of atoms is still too small for a ribbon structure as in the solid body. The addition of an additional atom or molecule can change a lot. Often there are many isomeric cluster structures with closely spaced energy levels. Molecular concepts lose their usefulness. Here, too, surface physics still plays a very important role because of the large ratio of surface atoms to volume atoms in the cluster.
- n = 100–1000 atoms ( large clusters ): A gradual transition to the properties of the solid is observed, such as transitions in crystal lattices ( chromium , for example, has a transition from fcc (<490) to bcc (> 490) at around n = 490 ; these values have a fluctuation range of 100 atoms).
- n > 1000 atoms ( small particles or nanocrystals )
Some, but not all, properties of the solid have evolved. From around 50,000 atoms the properties have developed so strongly that one speaks of a solid from then on .
Classification according to the chemical bond
- metallic clusters : half-full band of delocalized electrons, examples: alkali metal clusters, Al , Cu , Fe , Pt , W , Hg clusters; each with n > 200 atoms, average binding energy: 0.5-3 eV
- covalent clusters :bond through electron pairs alignedby sp hybridization ; Examples: C clusters (the best-known examples are the fullerenes , see also the picture above. Carbon nanotubes and diamond clusters also belong to the carbon clusters); Si , Hg clusters; each with 30 < n <80 atoms, mean binding energy: 1–4 eV, Hg ≈ 0.5 eV
- ionic clusters : bond through Coulomb forces between ions , examples: ( KF ) -, ( CaI 2 ) clusters, mean bond energy: 2–4 eV
- Clusters with hydrogen bonds : strong dipole-dipole attraction, examples: ( HF ), ( H 2 O ) clusters, average binding energy: 0.15-0.5 eV
- Molecular clusters : Van der Waals clusters, plus weak covalent components, examples: (I 2 ) -, (As 4 ) -, (S 8 ) -, organic molecule clusters, average binding energy: 0.3–1 eV
- Van der Waals cluster : induced dipole interaction between atoms and molecules with closed electronic shell, examples: noble gas , ( H 2 ), ( CO 2 ), Hg clusters; each with n <10 atoms, mean binding energy: 0.001-0.3 eV
Structure of free clusters
Friedel's rule is of outstanding importance for the structure of free clusters :
- The cluster structure with the greatest number of nearest-neighbor bonds has the greatest bond energy and is therefore the most stable of all possible structures.
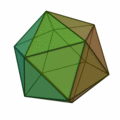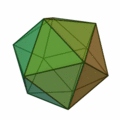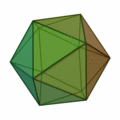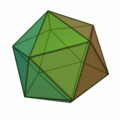
This rule usually results in structures based on the pattern of the Platonic solids :
The structure of the icosahedron is particularly important here (structure on the far right) , which occurs particularly frequently for clusters with an atomic number of n > 12. An atom is placed in every corner of the body and inside the icosahedral structure. Because of the 12 corners there are 12 surface atoms and 1 volume atom for a 13-atom cluster. For larger numbers of atoms, a new shell is filled with atoms to form icosahedral, wherein because of the greater binding length in the k th shell 10 k find ² + 2 atoms in the shell space. So there are 12 atoms for the first shell, there is space for exactly 42 more atoms for the second shell, 92 atoms for the third shell, etc. In all of these shells, like the k = 1 shell, there are 12 atoms on the corners of an icosahedron and an additional 10 k ² − 10 atoms on the surfaces. These concentric icosahedra must then be imagined as matryoshkas with the atoms on their surface. As in atomic physics with the stable noble gas atoms, there are also very stable clusters due to the shell closures. A closed cluster shell structure results only for a few cluster sizes: n = 13, n = 55, n = 147, n = 309, n = 561, n = 923 and n = 1415; they are called "magic numbers". These very stable clusters with filled atomic shells are commonly called Mackay icosahedra. The Platonic solids in the above figure appear in the microclusters, the hexahedron also in larger clusters.
The magic cluster numbers can be calculated using the following formula:
- = Total number of atoms
- = Number of atoms per edge
literature
Introductory literature
- H. Haberland: Textbook of Experimental Physics: Textbook of Experimental Physics 5. Gases, nanosystems, liquids: gases, nanosystems, liquids . Chapter cluster. Ed .: Ludwig Bergmann, Clemens Schaefer, Karl Kleinermanns. 2nd Edition. Gruyter, 2005, ISBN 3-11-017484-7 .
- Viktor Gutmann, Edwin Hengge: Inorganic Chemistry. An introduction . 5th edition. Wiley-VCH, 1990, ISBN 3-527-28159-2 (chapter cluster ).
Important publications
- AL Mackay : A dense non-crystallographic packing of equal spheres . In: Acta Crystallographica . tape 15 , no. 9 , 1962, pp. 916-918 , doi : 10.1107 / S0365110X6200239X ( Mackay icosahedron ).
- Jortner, Joshua: Level structure and dynamics of clusters . In: Reports of the Bunsen Society for Physical Chemistry . tape 88 , 1984, pp. 188-201 .
Individual evidence
- ↑ Oliver Rattunde; Hellmut Haberland: Clusterphysik . Retrieved January 19, 2017.
- ^ A b Erwin Riedel, Christoph Janiak: Inorganische Chemie . 9th edition. De Gruyter, Berlin 2015, ISBN 3-11-035526-4 , pp. 190 f .
- ↑ Helmut Haberland, Karl Kleinermanns, Frank Träger: Bergmann-Schaefer - Experimentalphysik Volume 5 - Gases, Nanosystems, Liquids - Chapter 9 Clusters . tape 5 . Walter de Gruyter, 2006, ISBN 978-3-11-017484-7 , p. 817 ff .