4 ', 6-diamidine-2-phenylindole
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
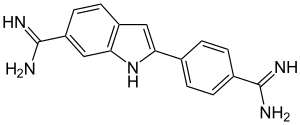
|
||||||||||
| General | ||||||||||
| Surname | 4 ', 6-diamidine-2-phenylindole | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 16 H 15 N 5 | |||||||||
| Brief description |
yellow, odorless solid (dihydrochloride) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 277.32 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
~ 330 ° C (decomposition) |
|||||||||
| solubility |
soluble in water |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
4 ', 6-diamidine-2-phenylindole , or DAPI for short , is a fluorescent dye that is used in fluorescence microscopy to mark DNA .
properties
4 ', 6-diamidine-2-phenylindole is usually marketed as di hydrochloride or its hydrate.
DAPI as a fluorescent dye
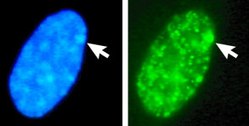
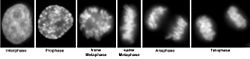
The compound preferentially attaches to AT -rich regions in the minor groove of double-stranded DNA. When excited with ultraviolet light , DAPI fluoresces in the visible range with a blue to cyan color. In connection with double-stranded DNA, the absorption maximum is at a wavelength of 358 nm, the emission maximum at 461 nm.
DAPI can also bind to RNA , which is found in abundance in most active cells. It intercalates into the RNA. However, the fluorescence is five times weaker than when it is bound to AT-rich DNA. The emission maximum is then at 500 nm. The emission maxima of DNA- and RNA-bound DAPI thus differ enough to be able to separate them with the help of optical filters and color splitters. DAPI is therefore used to display (only) DNA and, for example, to stain cell nuclei . This is not possible with many other nucleic acid dyes because the emission spectra of the DNA and RNA-bound dye molecules are almost identical. DAPI is therefore often referred to as “DNA-specific”, although, strictly speaking, this only applies to the emission at 461 nm (blue).
DAPI emits at the short-wave (blue) end of the spectrum of visible light. This means that further fluorescent dyes with longer-wave emission maxima can be used on the same preparation at the same time without signal overlapping ("bleed through"). For example, DAPI can be used simultaneously with green, yellow or red fluorescent markers such as Fluorescein , Alexa 488, GFP , Texas Red and many others. Due to the simple staining technique, DAPI is still one of the most important fluorescent dyes today. The ultraviolet radiation necessary for excitation creates radicals that also bleach other fluorophores. In some applications, DAPI is therefore the last dye to be stimulated and made visible in order to protect other markers. The fading of DAPI can be delayed by the use of anti-fading agents and the observation time at the microscope can be extended.
DAPI is able to penetrate intact cell membranes , but only very slowly, in contrast to the DNA dye Hoechst 33342 , which has similar spectral properties and also stains living cells quickly. DAPI is therefore rarely used for staining living cells. In addition, the high-energy ultraviolet radiation required for stimulation is harmful to living cells. Because of its DNA-binding properties, it is toxic and mutagenic .
DAPI also binds to polyphosphates, RNA, polyadenylate, some inositol phosphates, heparin and amorphous calcium phosphate with a redshift in higher concentrations .
literature
- Wilson, WD, Tanious, FA, Barton, HJ, Jones, RL, Fox, K., Wydra, RL, and Strekowski, L. (1990). DNA sequence dependent binding modes of 4 ', 6-diamidine-2-phenylindole (DAPI). Biochemistry 29, 8452-8461.
Web links
Individual evidence
- ↑ a b c d e data sheet 4 ′, 6-diamidine-2-phenylindole dihydrochloride (PDF) from Merck , accessed on December 25, 2019.
- ↑ External identifiers of or database links to 4 ′, 6-diamidine-2-phenylindole dihydrochloride : CAS number: 28718-90-3, EC number: 249-186-7, ECHA InfoCard: 100.044.700 , GESTIS substance database : 137000 , PubChem : 160166 , Wikidata : Q55972278 .
- ↑ Awtar Krishan and Payal D. Dandekar (2005): DAPI Fluorescence in Nuclei Isolated from Tumors. Journal of Histochemistry and Cytochemistry, Volume 53 (8): 1033-1036. doi : 10.1369 / jhc.4B6563.2005 .
- ↑ Tanious, FA et al. (1992): DAPI (4 ′, 6-diamidino-2-phenylindole) binds differently to DNA and RNA: minor-groove binding at AT sites and intercalation at AU sites . In: Biochemistry. Vol. 31, No. 12, pp. 3103-3112, PMID 1372825 .
- ↑ S. Omelon, J. Georgiou, W. Habraken: A cautionary (spectral) tail: red-shifted fluorescence by DAPI-DAPI interactions. In: Biochemical Society transactions. Volume 44, Number 1, February 2016, pp. 46-49, doi : 10.1042 / BST20150231 , PMID 26862187 .
