Diethyl aluminum chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
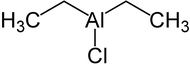
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Diethyl aluminum chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 4 H 10 AlCl | |||||||||||||||
| Brief description |
colorless and odorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 120.56 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.97 g cm −3 |
|||||||||||||||
| Melting point |
−74 ° C |
|||||||||||||||
| boiling point |
208 ° C |
|||||||||||||||
| Vapor pressure |
31 Pa (20 ° C) |
|||||||||||||||
| solubility |
violent decomposition in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Diethyl aluminum chloride is an organometallic compound of aluminum . It is used as a catalyst in the manufacture of chemical products.
use
Diethylaluminum chloride is used as a catalyst in Ziegler-Natta polymerization processes of vinylene , alkenes and dienes as well as linear oligomerizations and cyclizations of unsaturated hydrocarbons . It is also used as a catalyst in alkylation (see also Friedel-Crafts alkylation ). It is also used as an intermediate in the production of other chemicals.
safety instructions
Diethylaluminum chloride ignites spontaneously in air.
Individual evidence
- ↑ a b c d e f g h Entry on diethylaluminum chloride in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Hazardous Substance Fact Sheet: Diethylaluminium chloride , June 2001.

