Entecavir
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
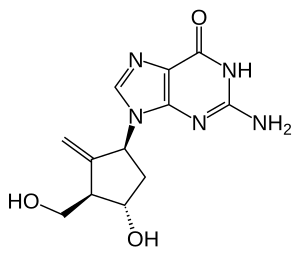
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Entecavir | |||||||||||||||||||||
| other names |
(1 S , 3 R , 4 S ) -2-Amino-1,9-dihydro-9- [4-hydroxy-3-hydroxymethyl-2-methylenecyclopentyl] -6 H -purin-6-one |
|||||||||||||||||||||
| Molecular formula | C 12 H 15 N 5 O 3 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 277.28 g · mol -1 | |||||||||||||||||||||
| Melting point |
> 220 ° C (entecavir monohydrate) |
|||||||||||||||||||||
| solubility |
slightly soluble in water (2.4 mg ml −1 at 25 ° C) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Entecavir is a chemical analog of the nucleoside guanosine . It is an antiviral selected from the group of nucleoside reverse transcriptase inhibitors ( NRTI ) and is used as drug (trade name Baraclude ® , Bristol-Myers Squibb ), for the treatment of hepatitis B used.
pharmacology
The nucleoside entecavir is phosphorylated to a nucleotide in the cell . Entecavir triphosphate is incorporated into the DNA in competition with the natural nucleotide deoxyguanosine triphosphate (dGTP). This leads to the inhibition of the viral reverse transcriptase :
- the primer formation by the HBV polymerase ,
- the reverse transcription of the negative DNA strand from the pregenomic mRNA ,
- the synthesis of the positive strand of HBV DNA.
literature
- Delaney, William E .; Yang, Huiling; Miller, Michael D .; Gibbs, Craig S .; Xiong, Shelly, Antimicrobial Agents and Chemotherapy , 3702-3710 (2004).
- Innaimo, SF et al., Antimicrob. Agents & Chemother., 1444-1448 (1997).
- Chang, T.-T. et al., N. Engl. J. Med. , 1001-1010 (2006).
Trade names
Entecavir is commercially available in Germany, Austria and Switzerland under the name Baraclude.
Individual evidence
- ^ The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition, 2006, p. 613, ISBN 978-0-911910-00-1 .
- ↑ Entecavir entry in the DrugBank of the University of Alberta , accessed November 28, 2018.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Rote Liste Service GmbH (Ed.): Rote Liste 2017 - drug directory for Germany (including EU approvals and certain medical devices) , Rote Liste Service GmbH, Frankfurt / Main, 2017, edition 57, ISBN 978-3-946057-10 -9 , p. 180.