Eriochrome black T
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
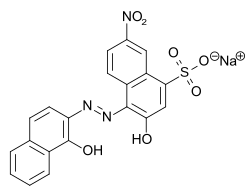
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Eriochrome black T | ||||||||||||||||||
| other names | |||||||||||||||||||
| Molecular formula | C 20 H 12 N 3 NaO 7 S | ||||||||||||||||||
| Brief description |
black solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 461.38 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| solubility |
soluble in water (50 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Eriochrome black T is an azo dye that is used as an indicator in complexometry and for determining water hardness . The intense color is due to the azo group and the water solubility due to the sulfonic acid group .
properties
Aqueous solutions of eriochrome black are wine red up to a pH value of 6.3. The sulfonic acid is deprotonated here and the two hydroxyl groups are protonated. By adding bases , the two hydroxyl groups are gradually deprotonated and the solution initially turns deep blue (dianion). If the pH value increases further, the color changes to orange at pH 11.5 (trianion). These indicator anions now form a weak purple-colored complex with bivalent metal ions , which is destroyed again by adding a stronger complexing agent such as EDTA . In order to create a greater contrast at the transition point, eriochrome black T is often used as a mixed indicator with methyl orange . This changes the color from red via a gray intermediate tone to green.
Structure of Eriochrome Black T at different pH values and the corresponding metal complex
Individual evidence
- ↑ a b Data sheet Eriochromschwarz T (CI 14645) (PDF) from Merck , accessed on March 30, 2011.
- ↑ a b Entry on Eriochrome black T in the GESTIS substance database of the IFA , accessed on February 10, 2017(JavaScript required) .
- ↑ Entry on Eriochrome Black T in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Hans Peter Latscha, Helmut Alfons Klein: Analytical Chemistry . Chemistry - basic knowledge III. 3. Edition. Springer-Verlag, Berlin, Heidelberg 1995, ISBN 978-3-540-58456-8 , pp. 288 ( limited preview in Google Book search).
- ↑ a b G.-O. Müller: Quantitative-inorganic internship , 7th edition, Verlag Harri Deutsch, Frankfurt / Main 1992, ISBN 3-8171-1211-4 , pp. 124-125.
- ↑ Lutz H. Gade: coordination chemistry . 1st edition, 3rd corrected reprint. Wiley VHC, Weinheim 1998, ISBN 978-3-527-29503-6 , pp. 241 ( limited preview in Google Book search).



