Ethoprophos
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
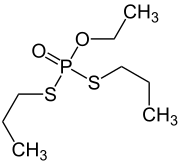
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethoprophos | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 19 O 2 PS 2 | |||||||||||||||
| Brief description |
yellowish liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 242.34 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.094 g cm −3 |
|||||||||||||||
| Melting point |
-13 ° C |
|||||||||||||||
| boiling point |
86-91 ° C (27 Pa) |
|||||||||||||||
| Vapor pressure |
|
|||||||||||||||
| solubility |
little in water (0.75 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Ethoprophos is an active ingredient for crop protection and a chemical compound from the group of thiophosphoric acid esters . The insecticide was discovered in 1966 and then introduced by Mobil Chemical . The active ingredient was later taken over by Rhône-Poulenc (now Bayer AG ).
Extraction and presentation
Ethoprophos can be obtained by reacting phosphorus oxychloride with 1-propanethiol (propyl mercaptan) and then reacting the intermediate product with sodium ethoxide . Alternatively, the reaction of phosphorus oxychloride with ethanol and subsequent reaction of the intermediate with 1-propanethiol is also possible.
properties
Ethoprophos is a yellowish liquid that is sparingly soluble in water. It is stable under neutral and slightly acidic conditions, but hydrolyzes rapidly under basic conditions.
use
Ethoprophos is used against nematodes and soil insects . This includes all weevils , flea beetles , cutworms ( cutworms ) such. As cutworms , beetle ( wire worms ), and more. The effect is based on the inhibition of acetylcholinesterase .
Admission
In some EU countries, Ethoprophos is approved as a plant protection product , but not in Germany, Austria and Switzerland.
Web links
- WHO / FAO Data Sheet on Pesticides (PDS) for Ethoprophos ( Memento of March 24, 2015 in the Internet Archive )
Individual evidence
- ↑ Data sheet Ethoprophos at Sigma-Aldrich , accessed on May 19, 2017 ( PDF ).
- ↑ a b c d e f g h Entry on Ethoprophos in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c Datasheet Ethoprophos ( Memento from February 8, 2014 in the Internet Archive ) from the Central Agricultural Pesticides Laboratory.
- ↑ Entry on Ethoprophos in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed on February 26, 2014.
- ↑ Entry on Ethoprophos in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide synthesis handbook . 1996, ISBN 978-0-8155-1401-5 ( page 353 in the Google book search).
- ^ A b Terence Robert Roberts, DH Hutson: Metabolic pathways of agrochemicals . Royal Soc of Chemistry, 1999, ISBN 978-0-85404-499-3 ( page 299 in Google book search).
- ↑ Mocap supplement ( Memento from March 5, 2014 in the Internet Archive )
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Ethoprophos in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on March 12, 2016.

