Extremity development
The limb development is an area of embryogenesis and forms a basis for insights into the molecular and cellular mechanisms for form-finding in the organogenesis of vertebrates. The development of extremities aims to explain the progressive formation of the skeletal patterns of the limbs in the tetrapods (land vertebrates with four extremities).
Overview of the overall process
The extremity begins to develop in the extremity field. The extremity field is a region in the right and left flanks of the embryo. The development of the extremities begins when the as yet undifferentiated, uniform mesenchymal cell tissue of the lateral mesoderm grows out of the somites and the ectoderm above it turns into a paddle-shaped extremity bud. The formation of an organizer, the apical ectodermal marginal ridge (AER), is a long, bead-like structure at the flattened tip of the bud, which controls the further growth of the bud in the direction away from the body (Fig. 4, 8b). Cells in the bud first condense, then differentiate in many steps into cartilage tissue, the chondrogenesis, and in its early phase form genetic and epigenetic patterns that later form the bones of the upper arm / thigh (Stylopodium), forearm / lower leg (Zeugopodium) and hand / foot (Autopodium) form. The exact location and form finding of these discrete, skeletal elements within the growing bud are the actual subject of research on extremity development. Towers and Tickle said in 2009: “Our knowledge of this process is still quite fragmentary and many of the proposed models remain controversial”.
In the following, models are outlined that have emerged since the late 1960s and provided molecular insights first into the development of the chicken wing (Fig. 2) and later also into the front extremity of the mouse (Fig. 1a, 1b, 3). Models are also listed (including the morphogen gradient model) that may be considered obsolete but still appear in newer textbooks. After more than 50 years of research, it has not been clarified in what form cells in the bud can receive position information that allows them to differentiate at precisely determined locations in cartilage tissue and thus enable the formation of patterns in the vertebrate extremity. It can be assumed that such position information does not exist. Newer computer simulation models, which operate on the cell level and not on the genetic level, work without position information and instead on Turing or Gierer-Meinhardt-based pattern formation processes. The challenges today therefore lie in the integration of molecular models with epigenetic (cellular) pattern formation with the help of computer simulations.
Terms (phenotype)
Important phenotypic terms for understanding the extremity and its development using the example of the front limbs of humans are:
- Upper arm (stylopod) (Fig. 3)
- Elle (ulna) and spoke (radius) = (Zeugopod) (Fig. 3)
- Hand (carpals and metacarpals) with fingers (digits) = (autopod) (Fig. 3). The autopod is the real vertebrate innovation in the limb.
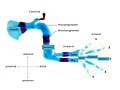
- AER Apical ectoderm marginal ridge (Fig. 4, 8b): Organizer region (signal center) for proximo-distal formation of the extremity bud
- ZPA zone of polarizing activity (Fig. 5): organizer region at the posterior end of the extremity; responsible for the antero-posterior axis formation. Significantly influences the number and identity of fingers.
- Morphogen : diffusible molecule that defines an organizer region. It forms a concentration gradient and directly or indirectly produces clear cell responses at different concentrations. Examples are retinoic acid (RA) or Sonic hedgehog (SHH).
Times and duration of limb skeleton formation
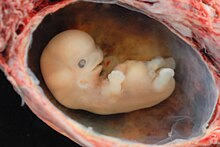
The development of the chickens' extremities, later wings and legs, takes about the third to sixth day, with a total development time of 21 days. In the mouse, the protrusion of the extremity bud begins after about 9.5 days; the bone formation of the extremities is completed four days later around the middle of the 13th day of development (Fig. 1b), with a total development time of approx. 21 days. In humans (Fig. 6), limb development begins in the fifth week of pregnancy (embryo: 6–8 mm). The hand development precedes the foot development by about two days. In the eighth week, arms and hands as well as legs and feet are largely developed (embryo: approx. 3 cm); this is followed by apoptosis , the death of cells, with which the fingers and toes are separated from each other individually.
Axial patterns and periodicity
The following main axes are distinguished in the extremity using the example of the human hand:
- Proximo-distal axis: from shoulder to fingertip
- Anterior-posterior axis: from the fifth finger to the thumb
- Dorso-ventral axis: from the inside of the hand to the outside
The extremity develops primarily along the proximo-distal, antero-posterior and the dorso-ventral axes. The processes along these three axes are partially, but by no means independent of one another. The axes are formed through coordinated interactions between signal centers. These are the AER (apical ectodermal ridge), which controls the proximo-distal growth, the ZPA (zone of polarizing activity) for the antero-posterior axis and the ectoderm of the bud, which controls the dorso-ventral axis. The growth of the bud in the proximo-distal direction is much more pronounced than in the anterio-posterior direction. Models either primarily deal with the proximo-distal development process, such as the progression zone model, or the antero-posterior process such as the morphogen gradient model. The integration of both axes in models is not yet very advanced.
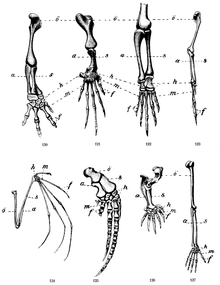
The extremity is divided into three regions proximal to distal: upper arm (English stylopod, Latin humerus), forearm (English zeugopod, Latin antebrachium) and hand / foot, autopod (Latin autopodium). In the distal direction, the bones are doubled and then multiplied. The stylopod consists of one, the zeugopod of two bones (ulna and radius). The autopod has three (chicken wing) to five (mouse) elements along the antero-posterior axis. Fingers and toes also show quasi-periodic patterns in the form of the tandem-like limbs along the proximo-distal axis. The elements at the same level of the proximo-distal axis have different identities, such as the ulna and radius, but also the individual fingers or toes.
The role of the Hox genes
In 1989 and 1991, Denis Duboule et al. Reported for the first time that the same homeotic system, whose relevance for vertebrates had only recently been worked out, is used several times in the organization of structures along different axes, especially in vertebrate extremity and in the urogenital system . The extension of this work to birds together with Cheryll Tickle and Lewis Wolpert demonstrated both the high degree of inter-species conservation and the essential role of the Hox genes in limb development. In 1991 Duboule published his "Concept of Posterior Prevalence". It stands for the functional organization of the Hox genes. In 1993 it could be shown that Hox genes are involved in multiple axial structures. It was possible to demonstrate this via a mutation of a single Hox gene that resulted in massive changes in the extremities, the axial skeleton.
Concepts of “regulatory landscapes” or “archipelagos” emerged. A large series of alleles made it possible to uncover the mechanism underlying colinearity in the development of vertebrate extremity. In fact, the axial organization of our legs and arms corresponds to the linear organization of regulatory chromatin domains. The transition between these chromosomal domains corresponds to the wrist, i.e. the transition between the evolutionarily old part (arms and legs) and the new part (hands and feet) of our limbs compared to the fish fin .
Deterministic models
The classical models of extremity development and their successor systems are based on the solution of the problem of pattern formation of genes and gene regulation and thus on deterministic processes. Form formation should be explained by the lowest, the molecular biological organizational level, as a hierarchical process chain. Today one wants to increasingly analyze the interactions of the various signal paths and understand their downstream effects. Rabinowitz and Vokes (2012) provide an overview of the current state of research on the integration of transcription networks in the vertebrate extremity. The most important models based on gene regulation are compiled by Towers and Tickle (2009).
The two necessary properties that the models in this group possess are the presence of a gradient and cell information. The cell can interpret the chemical signals of the gradient, which vary in strength or duration, and react specifically to them. It receives specifically recognizable cell information from the gradient. Based on this ability, it can differentiate into different tissues, such as bone, cartilage, muscle or connective tissue.
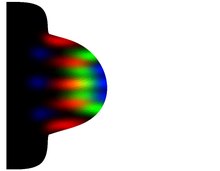
Morphogen gradient model (French flag model) according to Wolpert 1969
The morphogen gradient model was proposed by Lewis Wolpert in 1969 as a model for limb development in chickens. Wolpert postulates that the concentration of a morphogen that was not yet known at the time, segregated from the ZPA site of origin and spatially decreasing in the direction of expression along the AP axis, is responsible for the fate of the cells within range of this morphogen ( cellfate ) and thus for finger formation .
The morphogen forms a gradient and in this way supplies the coordinates or position information for the cells so that they can differentiate into cartilage tissue at precisely defined locations and thus the finger or toe patterns are formed. The diffusible protein Sonic hedgehog (SHH) was later identified as the likely morphogen. Wolpert assumed that the decreasing morphogen concentration is interpreted with threshold effects , i.e. as discrete gradations. He marked such threshold levels in blue-white-red, which gave the model the name French flag model (Fig. 7). Wolpert initially explained the mode of action of the morphogen by means of spatial concentration, but was later also expanded to include the temporal concentration of SHH in the direction of the antero-posterior axis, which, however, changed Wolpert's original model. The gradient acts on the cells in a way that has not yet been clarified. According to the theory, you can interpret its concentration and duration as position values, molecularly encode and finally translate it into the required finger anatomy.
The basic problem in Wolpert's model can be seen in the fact that one problem (position information) is explained by another problem (morphogen) for which no explanation is provided. Second, the model does not explain how a local, self-amplifying source for the morphogen can be generated. This explanation is only provided by Gierer-Meinhardt in 1972. Thirdly, in this morphogen gradient model, the fingers should actually be arranged in a circle around the ZPA, which is itself a three-dimensional, oval structure. The fingers are however lined up in one plane. The model cannot represent that. Fourth, the direct mode of action of a morphogen for generating exact position information originally propagated in the morphogen gradient model has recently been questioned as the sole explanation, and not only by Wolpert himself: In his more recent view, a morphogen cannot sufficiently reliably measure its precision and robustness generate both of which are required for pattern formation. The coordination processes have to be much finer. Fifth, signals for position information, as Wolpert imagined in his model, have not yet been found. For the reasons mentioned, this model can be considered obsolete from today's perspective.
Progression zone model according to Summerbell and Wolpert 1976
The classic progression zone model was introduced in 1976 as an extension to the morphogen gradient model (Fig. 8). Simply put, according to this model, the structures of the Styloped, then the Zeugopod and finally the fingers or toes emerge one after the other. The specific cell and tissue formation is to be explained by the fact that as the bud proliferation along the proximo-distal axis by the AER cells in the adjacent growth zone, they are initially kept "neutral", i.e. do not or do not differentiate. Depending on whether cells remain in the growth zone for a shorter or longer time until they leave it, different fate decisions are made about their fate: the later cells leave the growth phase, the more distal structures arise from them. The cells are therefore assigned an "internal clock" with which they can measure their whereabouts in the progression zone. The progression zone model is thus the first model that brings the factor time in connection with morphogen signals. This model is confirmed by removing the AER at an early stage, which leads to the absence of large parts of the stylopod and zeugopod, i.e. only forming a stump of the arm, while removing these parts at a later stage of development, but not the autopod and therefore not the fingers. The mechanism of action for the creation of the proximo-distal structures wants to explain the progression zone model with the autonomy of the still undifferentiated cells in the progression zone.
The progression zone model is not able to e.g. B. to explain the regeneration of a salamander leg. The clock would have to be reset after the leg was cut off. The model was also recently called into question by two studies that deny the cells said autonomy, at least the autonomy to generate the proximo-distal structure based on the assumed "internal clock". The authors, who independently came to comparable empirical results, explain the progression of the proximo-distal structure not with cell autonomy, but with opposing cell signals, the proximal retinoic acid (RA), which sends in the bud in the distal direction, and distal FGF activity, which works in the opposite direction starting from the AER. According to the authors, there is a dynamic equilibrium between these signals. This controls the proximo-distal formation of the Zeugopod and the Stylopod with the help of the appropriate Hoxgene. The researchers succeeded in z. B. to create desired distal structures in vivo by recombinantly transplanting proximal tissue to locations that have not been exposed to RA expression. Conversely, a complete proximo-distal axis with distal tissue could be generated in vitro by exposing the cultured cells to exogenous RA influence that overdriven the distal signals.
Multi-stage gene activation according to Meinhardt 1983/2009 and proximo-distal differentiation front model according to Tabin and Wolpert 2007

As early as 1982/83, Hans Meinhardt pointed out that cells cannot be transported to a more proximal or more distal specification, not all at once, but step by step, irreversibly. Gene activation was already seen as a space-dependent process. Then there is a feedback from the reached proximo-distal state of the mesenchyme cells to the strength of a posteriorizing signal that is formed in the ectodermal AER. It has been assumed that a rudimentary pattern is initially formed (e.g., 1112 when the sequence of the complete proximo-distal structures is labeled 1-6). If enough cells of this type are formed through the proliferation of type 2 cells, the signal produced in the AER increases to such an extent that cells of type 3 are formed. Only when enough type 3 cells are formed are type 4 cells formed (Fig. 10).
This model is compatible with the progression zone model. It is a feature of the model that after an amputation the missing structures can be formed again at any point, hence the name Bootstrap model . Furthermore, the model allows that distal structures can be formed too early, e.g. B. when the spread of the molecules is disturbed by dead cells. Such a pattern corresponds to the thalidomide malformation. Meinhardt refined his theory in 2009: “A higher organism is far too complex to be generated by a single morphogen gradient. Only gene activations that proceed in a switch-like manner one after the other, with the help of a gradient, gradually form ever sharper boundaries and positions for the skeletal form: The consequence of the chronologically graduated gene expression records are ever finer pattern and position boundaries. The boundaries between regions in which different genes are active can become new organizing regions in order to form the patterns of limb sections ”, in this case that would mean: to define regions of limb sections such as the upper arm, forearm or fingers or toes.
Such limits could e.g. B. be the different ways of reacting to SHH (concentration, duration or both together) that develop between the toes of the mouse, as described by Harfe et al. 2004. Position information that is transmitted to the cells in the classic Wolpert sense by the concentration and / or temporal effect of a single morphogen as unambiguous coordinates no longer exists here. Rather, the morphogen activates several stepped expression processes one after the other, in the course of which clear positions and phenotypic courses emerge.
For Tabin and Wolpert, the progression zone model does not do justice to the large amount of molecular gene expression data that has been generated in recent years. An example of this is the influence of Hoxgenes, which has not been included in the previous models. The authors therefore presented an alternative framework for proximo-distal patterning, taking into account the available data. According to this, there are several different gene expression domains, each with specific groups of interacting genes in successive phases of bud growth. A differentiation front is formed at the proximal limit from which AER-FGF signals can no longer be received. This boundary prefigures the proximo-distal sequence through which the individual elements gradually appear in chondrogenesis. The result is the creation of several progenitor pools for each segment (Zeugopod, Stylopod and Autopod). The differentiation front model thus describes very similarly what Meinhardt already presented earlier.
Self-regulating system of interdependent cell signal feedback loops according to Bénazet, Zeller et al. 2009
Bénazet, Zeller, et al. University of Basel, claim to present the first integrated model that links known and new signaling pathways in positive and negative epithelial- mesenchyme feedback loops and thus integrates existing models. However, this applies to earlier models, e.g. B. that of Tabin and Wolpert 2007, already. The team around Zeller shows a threefold system, consisting of an initiation phase, an expansion phase and a termination phase. Gremlin1, its antagonist BMP, FGF and SHH are the pillars of the three subsystems. In the initiation phase, the model starts before the earlier models and first explains the prerequisites for AER and ZPA to be able to take up and maintain their function. The authors speak of prepatterning in this phase, whereby position information is "checked" at an early point in time and continuously updated in the course of growth. Their exact fixation takes place only in later development phases, but before the differentiation of the cells. Threshold values as in the morphogen gradient model are not required for this.
The model is understood as a systemic approach. However, the spatial aspect is not addressed very much. A general problem in understanding how leg structures are formed is that there are few site-specifically activated genes. For this reason, the model does not reveal at all how the fingers are formed in space-time. A simulation has not yet taken place and is also not possible. The BMP receptor model developed at ETH Zurich in 2012 goes much further here. It is noteworthy, however, that both models, which were even developed in cooperation between the two teams, and which both want to explain the early phase of the structure formation of the extremity, cite different control loops for the same situation. The Shh signal path, for example, which has a high priority in more than one phase in the Zeller team, is seen by the other team as dispensable with reference to Litingtung et al. 2002.
Autoregulatory models
The evolutionary developmental biology looks patterning processes not primarily on the genetic or Genregulationsebene but in interactive interaction of genetic with higher organizational level (cells, tissues). The work of Alan Turing (1952) is fundamental to all of the models listed below. as well as by Gierer and Meinhardt (1972). Turing mechanisms can produce irregular patterns; But only the dynamic processes with self-organization and scaling described by Gierer and Meinhardt are the epigenetic mechanism for regular morphological form-finding, including of the extremities. Early on, parallel to the gene-centered approaches, epigenetic possibilities of extremity development were pointed out. More recent models are no longer based on chemical diffusion processes, but rather on cell-cell reactions and can use this as a basis to map biological activator-inhibitor processes more realistically. Regardless of this, there are still considerable difficulties to reproduce the activator-inhibitor processes in an adequate molecular-empirical manner. A number of such models are presented below. The first three are further developments of the mathematically comprehensive LALI model by Hentschel et al. 2005. LALI stands for the extended Turing model type Local auto-activation, lateral inhibition. in the sense of Gierer Meinhardt.
Self-organizing properties of mesenchymal pre-cartilage tissue - Newman and Müller model 2005
Newman and Müller contrasted the classic models with a new view of the creation of the hand (origination and innovation), which, in addition to the genetic, epigenetic processes and the interaction of both, comes into focus (Fig. 11). A handful of cellular and molecular core processes of the mesenchymal tissue form the basis for the processes. Existence and transmission of position information in individual cells are not required. This approach provides a systematic approach for the first time in that the interdependent interaction of the system components genes, gene products, cells and tissue is treated. According to this view, epigenetic processes are fundamental and indispensable not only for explaining the evolutionary origin of the hand ( origination ), but also for explaining its development in recent living beings.

Three central properties of the epigenesis of the development system are formulated:
- the autonomy properties of their components (cell behavior, tissue geometry, etc.),
- the ability of the mesenchymal tissue to self-organize during condensation and to develop spatiotemporal patterns,
- the non-linear responsiveness of cells and tissue to minor changes such as genetic mutation, changes in epigetic parameters or environmental influences. Exceeding ( thresholds ) may be responsible for discrete phenotypic variation or innovation .
The model by Chaturvedi, Hentschel, Alber, Newman 2005
This model uses computer simulations that can map the organogenesis of the hand based on cell and cell communication processes with reaction-diffusion equations and, for the first time, capture the development of fingers in three dimensions (Fig. 12). The model is based on the original, but modified, reaction-diffusion equation set by Hentschel et al.
The following model sizes and methods are used for spatiotemporally regulated condensation in the Hentschel model:
- 1. 3D cell arrangement
- 2. Cellular Potts Model (CPM), the very core of the model; a simulation protocol that describes the spatiotemporal order in which the components (cells etc.) interact or adhere. The CPM simulates the collective behavior of cell structures. In the case here, it allows cells to behave. The CPM is a generalized Ising model . The Ising model comes from theoretical physics and explains the spontaneous phase transition from the non-magnetic to the magnetic state of solids. It has been adopted into many other areas of science. In the case here, the physical formalism for investigating the implications is that cells, depending on the cell type, have different degrees of adhesion to one another. It describes the modeling of cell dynamics taking into account minimal, fluctuating energy input. (The CPM is used here in the open source computer program CompuCell3D, a simulation program for complex biological problems.)
- 3. Pattern-initiating Turing instabilities of excitable media on the levels of tissue, organs, organism.
- 4. Morphogenic fields as non-molecular continua.
- 5. Interaction of different biological organizational levels (scales) through further modular standard computer programs with defined interfaces; Flow of information from finer to coarser structures or within pairs of modules. The explicit levels are:
- Molecular level ( cell nucleus ),
- Subcellular level ( mitochondria ),
- Cell level,
- Cell and tissue level,
- Tissue level,
- Organ level
- 6. The corresponding mathematically formulated mechanisms that the model uses at these levels are (starting again from the molecular level):
- Production of morphogens TGF-ß, FGF-2 and FGF-8; TGF-ß produces threshold effects;
- Generation of a fibronectin field ,
- Diffusion of extra- and intracellular chemical substances through reaction-diffusion equations ,
- Gene regulation ,
- Regulation of cell adhesion ,
- Cell dynamics and response to morphogenic fields,
- Cell membrane fluctuation ,
- Cell growth ,
- Mitosis (cell nucleus division),
- Apoptosis (programmed cell death),
- Cell differentiation ,
- cell-specific mobility between cells and between cells and the extracellular matrix,
- Haptotaxis (no chemotaxis),
- extracellular matrix through which morphogens diffuse (cell fluid),
- as well as at the top level: thermodynamics and mechanics of condensed tissue.
The Chaturvedi model is based on relatively few molecular processes, but uses a variety of cellular and supra-cellular mechanisms. With this procedure it can represent rough skeletal, spatial structures. We are working on the integration of the mode of action of Gli3, Wnt-7 and Shh, which control the extremity geometry and zones, which will lead to a further realism of the models. Organizer regions in the bud, such as AER and ZPA, but also the growth zone are highlighted a priori. Their origin is therefore not reproduced. Their specific location in the bud does not matter in the model. For reasons of computing capacity, this model is not primarily based on molecular processes. Its inner core is the cell with the CPM software application.
The model by Zhu, Zhang, Alber, Newman 2010
In 2010, a simulation model was published that provides expanded knowledge of extremity development and can also show evolutionary differences.
Like the previous one, the model is based on the model by Hentschel et al. (2005), and is therefore also based on activator-inhibitor equations and thus on the ability to organize itself outside of genetic information in the mesenchyme. It does not require any position information a priori. Activators and inhibitors are comparable to the model by Newman and Müller (2005). Zhu et al. Do not go into the problem that up to now no single morphogen can be empirically identified as an inhibitor and that a little before the molecular identity of a long-range inhibitor was described as elusive. An inhibitor must be assumed, otherwise the model cannot be represented. The focus of this model is a fibroblast growth factor (FGF), a gradient as an important proximo-distal player based on the AER. Furthermore, BMP activity is dictated by underlying molecular “prepattern”, through which the cellular pattern is configured. The basic diffusion process for patterning is only determined by such a multi-stage process, which is not yet transparent at the molecular level.
In the simulation model (see video Fig. 13), growth and condensation follow the progression zone model. The AER (far right), a narrow progression zone (middle) and the growth zone can be clearly identified.
The model generates two-dimensional patterns and, compared to the models by Hentschel, Newman-Müller and Chaturvedi, is further simplified in terms of the number of equations and reduced to two reaction-diffusion equations. Progress is being made in that the different zones of the bud such as AER, frozen zone, growth zone are differentiated. Different cell processes that run empirically are incorporated or kept constant in the model. An expansion of the AER as the bud grows is included. A curved apical contour is assumed. In general terms, the model takes into account the reshaping of the bud tissue.
Compared to the previous models, asymmetries can be represented in the autopod, ie differences in the skeletal elements with regard to the number of toes or their length, as they actually occur empirically in different species. Shh and Hox activities are taken into account. Evolutionary transitions can be created. The model results of this model do not arise on the lower, genetic organizational level. Rather, the Turing equations reflect the self-organization of the bud cell tissue, i.e. autonomous properties that only come about at this level. Hoxgene, gene regulation, especially transcription factors are decisive for the parameters of the equations. In this way they control and refine the spatial alignment of the bones, but they are not the actual shaping factors. These are epigenetic. The evolutionarily or species-specifically different length, thickness and alignment of the bones is achieved by means of changes in so-called kinetic reaction parameters, which represent the production rates of the activator morphogen or which stand for the association rate constant of activator and inhibitor.
Zhu et al. Simulate both the proximo-distal outgrowth of the bud and the antero-posterior pattern formation of the autopod, ie the number of toes. These correlate positively with the increasing width of the autopod. To widen the autopod, a parameter is used that can be understood as a simulation of the Shh expression of the ZPA. The simulation, based on continuous changes at the gene and cell level, thus forms discrete elements.
One can critically evaluate the sharpness of the bone formation on the model, which in the simulation (Fig. 13) emerges directly from the progression zone, is not very realistic in comparison to the older Fig. 2, which is also from Newman. The specification process of the bones (cartilage formation) is more gradual. The toes are not formed at the same time (Fig. 2). It should be positively emphasized that the Zhu model contains both a proximo-distal and antero-posterior growth and the latter also allows polydactyly forms as part of the broadening of the bud.
BMP receptor interaction model from Badugu, Iber et al. 2012
A new model comes from the Department of Biosystems Science and Engineering at ETH Zurich. For the first time, the following approaches are integrated here: (A) A Turing model (rather LALI model) with molecular, BMP-receptor-based interactions and FGFs, (B) a growing domain, (C) a realistic 2D bud geometry of the Mouse. The model starts with the BMP signal path at the earliest recognizable interface for the onset of condensation. Shh, Gli and Gremlin are not needed in the model at all. The simulation is compatible with a large number of empirical experiments on the bud as well as with several pre- and postaxial polydactyl mutants. It is noteworthy that the statements in this model are not clearly comparable with the model by Bénazet, Zeller et al., Which was created in 2009 at the University of Basel. Furthermore, the pattern image generated in the model does not resemble the empirical image of prechondrogenation of the vertebrate hand as in Fig. 2.
Hox genes regulate the spacing and number of fingers in a 2012 Turing model
Another new model places Turing processes on the previously unused basis of the Hox genes . So far, only gradients have been seen in Turing models, i.e. diffusing substances that act as activators or inhibitors. It is therefore a new view that non-diffusing transcription factors such as the Hoxa and Hoxd groups play a decisive role in pattern formation. This is exactly what Sheth et al. Demonstrated empirically in 2012. According to this, the expression of these genes, which are expressed late distally in the development of the hand, is inversely proportional to the number of fingers: Their reduction increases the number of fingers. If the reduction occurs in parallel with the reduction of Gli3R, polydactyly is even more pronounced. The mentioned Hox genes are seen to be responsible for the wavelength of the finger spacing. Under the conditions mentioned, up to 14 fingers can be generated on one hand empirically with the mouse and in the model.
The BSW Turing model by Raspopovic, Marcon, Russo, Sharpe 2014
The latest Turing model for vertebrate hand development comes from a team at the University of Barcelona (Chair of Multicellular Systems Biology : James Sharpe ). It was published in Science Magazine in August 2014. The model propagates the interaction of Bmp, Sox9 and Wnt (BSW model). This is an approach that has been empirically verified on a large scale with regard to the genetic actors mentioned. Each individual factor was individually checked for its effectiveness in the model using a knock-out procedure. The Turing model suitability of the influencing variables was also determined comprehensively mathematically and the robustness of the model ensured that the desired number of five stripes (representing toes) was always created. The model is two-dimensional. It is limited to the pattern formation in the autopod (fingers and toes), because here, because of the repetitions of the toes, in contrast to the Zeugopod (e.g. upper arm) and Stylopod (e.g. forearm), the better plausibility for a Turing mechanism is seen.
The BSW model uses three main actors as opposed to two factors each in previous Turing or LALI (activator / inhibitor) models: Bmp, Wnt and Sox9 (Fig. 14A). Bmp and Wnt are morphogens, Sox9 is a transcription factor. The effect of these genes in limb development has already been described. For the first time, however, an overall connection is established that Bmp has a self-reinforcing (auocatalytic) effect, Bmp activates the expression of Sox9, Sox9 inhibits Bmp and Wnt and Sox9 also inhibit each other (Fig. 14A). Sox9 is expressed in the toes throughout the condensation process. It has therefore been used for a long time as an ideal gene marker in order to be able to empirically follow the gradual chondrogenesis of the extremity. The expression of this gene is shown in the model in regular waves (FIG. 14C). These waves represent stripes in the two-dimensional Turing model; these represent the toes (Fig. 14B, d2-d4). The wave movement of stripes and their spaces is justified by the fact that whenever Sox9 is active, Bmp and Wnt are suppressed (finger spaces) and vice versa, i.e. waves running opposite to the Sox9 expression pattern build up (Fig. 14C). These two wave patterns in the model, Sox9 versus Bmp / Wnt, empirically represent the alternation of the toes and their spaces.
Without further parameters, the model only generates randomized strips that run roughly in the proximo-distal direction, but do not show a stable number and orientation. The stripes should also appear radial, as can be empirically observed in the mouse (Fig. 1a, 14B). Raspopovic argue with Hoxd13 and Fgf's as stabilizing parameters. Both genes are expressed distally in the bud. Since the bud has a rounded, distal shape, the Fgf's expressed in the AER act in the direction of growth of the toes. In contrast, the distal expression of Hoxd13 - initially posteriorly in the vicinity of the ZPA and increasingly expressed over the entire antero-posterior width - ensures that the distances between the stripes become increasingly wider in the course of growth, as shown earlier. Both processes together, Fgf's and Hoxd13, stabilize the Turing model according to Raspopovic and others, and the arrangement of the stripes in the model takes on the desired radial shape. In summary, the aforementioned factors Bmp, Sox9, Wnt with the control parameters Hoxd13 and Fgf's generate an interesting, empirically substantiated scenario of vertebrate extremity development.
It must be seen critically that although it has been empirically confirmed that Hoxgenes are only expressed late in the bud, they do not necessarily have an expression pattern with which they can play the role that the BSW model assigns them. As with all previous models, the model cannot simulate cell compression either. All models work, even if with growth of the bud, at least with a constant number of cells per unit area. Cell condensation is an elementary, easily observable and confirmed phenotypic process in the developing extremity.
Critical appraisal of the models from the present point of view and future research priorities
For a long time, with the two classic models, the morphogen gradient model and the progression zone model, separate considerations on the structure formation of the hand existed, on the one hand the explanation by the processes along the proximo-distal axis, on the other hand by the explanation that was initially independent of it the ZPA processes along the antero-posterior axis. However, none of the models can adequately explain the spatial formation of identities in the Bud, and especially in the Autopod, on their own. Each of the models is confirmed by empirical cut-and-pace tests. Ultimately, however - if one follows the logic of the models mentioned - the coordinates can only be determined exactly when the cells in question have their location on the proximo-distal axis as well as that on the antero-posterior axis and, strictly speaking, also that on the dorso-posterior axis. ventral axis can be communicated. From this point of view, there must be processes in the embryogenesis of the hand that make it possible for cells to interpret position points on all three axes.
The persistent difficulties in discovering position signals empirically led other scientists to the more holistic, systemic explanation of structure formation, at least in the direction of the two main axes. The further developments in this area are characterized by the focus on interdisciplinary, computer-aided ( in silico ) research. The challenges lie in the following areas:
1. Improved synthesis of molecular with systemic models.
2. Simulation of the behavior of the bud in the dynamic growth process. Both morphogens and reaction-diffusion systems behave differently in growing domains than in static ones.
3. Transition to stochastic compared to the previously often deterministic models with regard to gene expression, cell processes, etc.
4. Detection of the robustness of cell processes used, so their immunity to stochastic noise ( noise ).
5. Analysis of the scaling problem, d. This means that different bud sizes ( domains ) create different patterns. The strips in the French flag model do not scale evenly, nor does the same number of strips result in activator-inhibitor systems with an enlarged domain.
6. Empirical determination of the activator and in particular a long-range inhibitor for skeletal form-finding. Both parameters are indispensable in activator-inhibitor systems.
7. Empirical determination of the molecular basis for possible position information, if actually available ( receptors ).
8. Determination of suitable methods to make the distribution of SHH and other morphogens in the developing bud quantitatively visible in real time.
9. Integration of the dorso-ventral axis and its molecular-cellular basis as a prerequisite for empirically based three-dimensional models in which all the hand limbs lie in one plane.
10. When do joints form, if this is not the case only in the places where there is periodicity (elbow, knee, wrist / ankle)? The phalanges of fingers and toes are also connected by joints.
Methods of empirical research
The external location of the extremities allows a variety of manipulations on the embryo, the results of which are easily visible. In most cases, genetic changes do not lead to the death of the test animal. For these reasons, the vertebrate limb stands out as an excellent model system. The first few decades were primarily devoted to experimental transplant attempts on embryos. First, grafts were removed from the bud and replanted in other places. When the ZPA and AER and their positions were known, these organizer regions were taken from other animals and z. B. the ZPA anterior in addition to the posterior replanted. Furthermore, these components were removed and / or implanted at earlier / later times or in larger / smaller doses. It was hoped that this would provide information about changes in skeletal formation. More recently, in-situ hybridization and, above all, gene knockout have been used in molecular biology . By switching off genes, one can infer their function in the development of the extremity. One also speaks of gain of function or loss of function experiments. The experiment by Litingtung et al. In 2002 led to the surprising finding that a double knock-out of Sonic hedgehog and Gli3 (Shh - / -, Gli3 - / -) would produce a polydactyl hand that could not be expected. Both genes are therefore unnecessary for finger formation if they are switched off together. The experiments mentioned do not provide information, or only to a limited extent, about how important signal pathways interact and which downstream effects they generate. It is controversial whether the factors of phenotypic pattern formation (e.g. fingers) can be recognized in this way.
Sonic hedgehog - key gene for limb development?
No other gene or protein has been the subject of more research related to the development of vertebrate extremity than Sonic hedgehog . Hedgehog is a highly conserved evolutionary signaling pathway and plays a central role in a number of pattern formations in both vertebrates and insects. Countless experiments were conducted with the ZPA to determine its mode of action before it was known what the morphogen in the ZPA was. When Tabin discovered in 1993 that Sonic hedgehog is the morphogen that is expressed in the ZPA cells and diffuses into the extracellular matrix of the autopod from posterior to anterior, the focus on researching the mode of action of Sonic hedgehog became even higher. The proliferation of cells in the course of Shh expression and thus the anterio-posterior growth of the bud as a consequence of the ZPA became clear (Fig. 15). According to Tickle, SHH has now been confirmed as an important morphogen for pattern formation in the autopod. Its far-reaching effect depends on both the dose and the duration of the diffusion. The ZPA then checks both the number and identity of the fingers. Most hand malformations on the thumb side (preaxial polydactyly ) are related to changes in the expression of Shh.
Today it is no longer assumed that the diffusing mode of action of SHH can be sufficiently precise to be able to determine the position of the cells (cartilage formation and adjacent tissue or exact skeletal shape) on its own. It cannot be ruled out that SHH itself does not have a graduated effect, but that it induces the production of another substance that has the corresponding gradient effect and passes it on. Or there are several sequential patterning processes that refine structures or positions. The model by Badugu et al. (2012) for explaining digit patterning works without Shh, as does the BSW model by Raspopovic et al.
Sonic hedgehog was also one of the first genes to be found to have a highly conserved, non-coding cis regulatory element (ZRS) that controls Shh expression in great detail in both mammals and fish. Mutations in the CRS lead to preaxial polydactyly. After all, the Sonic Hedgehog signaling system was one of the first to be described as producing a stochastic genetic switch and thus threshold value effects in limb development in the regulation of Gli.
In addition to its antero-posterior effect in the autopod, Shh interacts with the AER via the ZPA and maintains its function permanently during the growth of the bud. Conversely, Shh is no longer expressed when the AER is eliminated. FGF4 acts to maintain Shh and Shh acts to maintain FGF4. Without this interaction, fingers are either reduced to the first finger or they are completely absent. However, this relationship does not mean that Shh is directly responsible for the number and identities of the distal elements, as seen by Tabin and McMahon in 2008. It is not even unequivocally clear whether a correlation exists between the duration of SHH signaling required to specify a finger and the order in which cartilage condensation occurs. From today's perspective, SHH primarily has two main functions: It controls the antero-posterior identity of the fingers and the number of cells and thus the growth of the bud. Threshold value effects of finger formation only occur indirectly via SHH. Intensive research is still being conducted into the mode of action and role of SHH for digit patterning.
Robustness versus plasticity of toe number and length
The vertebrate extremity is a very robust anatomical shape. The five-fingered system occurs in mice such as elephants. Since it cannot be assumed that the embryos of such different species are the same size at the time of extremity development, the development mechanisms relevant for further training must be independent of size. At the same time, the extremity is evolutionarily characterized by an extreme adaptability, as can be seen from their different shapes in vertebrate species (Fig. 16 and 17). Both properties seem intuitively contradictory. However, Andreas Wagner was able to show that robust forms with well-developed gene networks have better conditions for variation and innovation than less robust forms.
The pentadactyl upper limit of the hand (five fingers) has not yet been conclusively clarified. Because of the similarity of the toes, early types of dinosaurs (e.g. Acanthostega ) are now interpreted as polydactyl rather than seven or eighteen. There is no vertebrate species that normally has more than five fingers or toes on an extremity. The panda bear and mole have deformed metacarpal bones that are like fingers. Exceptions with selective human influence are certain populations of cats ( Maine Coon cats ) and the Norwegian Lundehund ( polydactyly ). Galis cites development constraints as the reason for preventing a larger number of fingers.
More recent research results exist on the extreme length of bat wings. At an early embryonic stage of development (day 16 after fertilization) and thus after the condensation and segmentation phase in the extremity bud, the toes of the forelegs are about the same length as those of the mouse on day 12.5 (see Fig. 1b). The lengthening of toes 3, 4 and 5 of the bat in particular from the 20th day (Fig. 16) could be associated with increased expression of the bone morphogenetic protein Bmb2. Bmp2 stimulates cartilage formation and differentiation and increases the toe length in embryonic development. The evolution of the bat is thus justified by changes in the development of length growth and the length differentiation of cartilage tissue in the front extremity.
Important proteins and their function in limb development
| Surname | function | description |
|---|---|---|
| BMP | Morphogen | Family of the bone morphogenetic proteins, belong to the TGF-ß signaling pathway. The BMP signal transduction pathway is involved in the development of fingers and hand and numerous malformations. BMP is necessary for the early induction of the form and function of AER. In Zhu et al. (2010), the BMP environment determines the lateral inhibitor. In Badugu et al. 2012, a BMP receptor interaction stands for the beginning of condensation. In the BSW model, BMP plays the central activator role. It is expressed together with Wnt in the finger spaces and activates the expression of Sox9. |
| β-CAT | protein | Component of the WNt3 / β-catenin signaling pathway. β-CAT is activated by Wnt3. A lack of β-CAT leads to severe defects in the development of the extremities: Dorso-ventral patterning in the early phase of AER training does not occur. Abortion of the front and complete absence of the rear extremity (see also WNT). |
| DKK1 | protein | Dikkopf-1, important Wnt3 antagonist in the early development of the bud. Overexpression causes limb growth to stop. Knockout of Dkk1 leads to malformations of the fingers (postaxial polysyndactyly) |
| dHAND | Transcription factor | (also Hand2), activates Shh. |
| FGF | Signaling pathway, growth proteins | Fibroblast growth factor protein family. FGFs induce the AER and are largely responsible for the formation of the progression zone proximally adjoining the AER, ie for cell proliferation and differentiation in the growing bud. Fgf8 is expressed continuously in the AER from a very early point in time and then throughout limb development. It controls Shh expression and vice versa. Fgf4 is another important Shh inducer. |
| GLI3 | Transcription factor | Member of the GLI protein family. Component of the Shh signaling pathway. In mice, Gli3 is associated with finger specification and polydactyly . Polydactyls through associated Gli3 point mutations. Gli3R prevents the expression of the activator form Gli3 and thus has a repressive effect on Shh; Combined exposure of Gli3 and Shh in the mouse is associated with polydactyly, just as the knockout of GLi3 alone Gli3 is largely responsible for the antero-posterior limb asymmetry due to the posterior Shh down regulation. GLI3R is the main antagonist of SHH. It is expressed as a morphogen opposite to SHH, strongly anteriorly and decreasing posteriorly. |
| GREM | protein | Antagonist of BMP. Important component of the SHH-GREM-BMP-FGF-Feedback-Loop for the amplification of the AER-FGF-Signalgenbung in the model Bénazet-Zeller 2009. |
| HOX | Transcription factors | Family of the Hox genes or molecules; many of them involved in basic structural and Symmetry development of the embryo, including the extremity. The Hoxgenes are arranged in 4 clusters (A – D) with up to 13 Hoxgenes each. They are expressed in a nested pattern along the proximo-distal axis. Hoxd11 and Hoxd13 are involved in finger formation; also Hoxa11 and Hoxa13. Knockout of the Hoxa13 specifically expressed in the autopod leads to deformations of the autopod, as does the double knockout of Hoxa13 / Hoxd13. Tabin discusses the strict sequence from Hoxd8 to Hoxd4 expression and Hoxa10 to Hoxa7 expression as decisive for the number of fingers. According to recent research, Hoxgenes are seen as regulators for a Turing mechanism. Switching off Hoxgenes increases the number of fingers up to 14 per mouse extremity. This is done by primarily not widening the bud, but narrowing the space between the fingers. For a Turing model that would be the wavelength that changes. Since Hoxgene are transcription factors for switching on other genes and not gradients, they have to regulate other substances that have the diffusing property. It is not clear which these could be. |
| RA | Morphogen, signaling pathway | Retinoid Acid (Retinoic Acid), Vitamin A. As a morphogen, it is not sufficient for finger formation or identity as originally assumed. The expression occurs from proximal to distal with decreasing concentration. The autopod is in the area of the lowest concentration. Its correct development depends on the balanced, weak, distal RA expression. RA can cause finger duplication ectopically and mimic an ectopic ZPA. RA positively regulates SHH and interacts with FGFs for proximo-distal length growth of the bud. |
| SHH | Morphogen, signaling pathway | One of 3 proteins in the Hedgehog signal transduction pathway, responsible for the number of cells in the autopod and identification of the fingers, only indirectly for the number of fingers, as well as being the cause of preaxial polydactyly, Shh interaction with Gli3 is fundamental for finger initiation and identification (Wagner & Larsson in Hall 2007, 55). SHH also controls the production of FGFs. One of the main functions of SHH is to keep in check the morphogen GLI3R, which rotates in the autopod from anterior to posterior. FGFs, TBX2, HAND2 and other proteins control the expression of Shh. SHH and FGFs interact and thus mutually maintain the functioning of the two organizing regions. |
| SOX9 | Transcription factor (protein) | SOX9 is expressed throughout the condensation process of extremity development. It serves as a gene marker for cartilage formation. Important player in the BSW model |
| TGF | Growth factor (protein) signaling pathway | Transforming Growth Factor Family. TGF-ß controls cell proliferation and differentiation. In the models Newman & Müller (2005), Newman & Bhat (2007), Zhu et al. (2010), TGF-ß is the activator environment. |
| WNT | Morphogen family, Wnt / β-catenin signaling pathway | WNT is an autoregulator component. WNT5 is stimulated by FGFs for directional growth of the proximo-distal axis in the distal direction. Absence of Wnt3 leads to abortion of the anterior extremity or the complete absence of the posterior extremity (see also β-CAT). WNT is one of the main factors in the BSW model. It is expressed in the spaces between the fingers, where it inhibits SOX9 expression, which is restricted to the finger areas. |
Milestones in the research of extremity development
| year | Researcher | discovery |
|---|---|---|
| 1808 | Louis Sébastian Marie de Tredern de Lézérec | Dissertation on the developmental stages of the chicken in the egg, including the first detailed description of the developmental stages of the chicken foot. |
| 1948 | John W. Saunders and Marry T. Gasseling | Identification of the AER in the chicken wing. Removal of the AER leads to complete cessation of bud growth and the absence of skeleton formation |
| 1952 | Alan Turing | Oscillating chemical reactions as development mechanisms, described in partial differential equations (Turing mechanism). Activator-inhibitor distinction, self-organization and scaling not yet made (cf. 1972). |
| 1968 | John W. Saunders and Marry T. Gasseling | Identification of the ZPA; Anterior Transplant of the ZPA; Duplication of fingers in the chicken (Fig. 16). |
| 1969 | Lewis Wolpert | Morphogen gradient model (French flag model). Spatial morphogen effect. |
| 1972 | Alfred Gierer and Hans Meinhardt | Introduction of activator-inhibitor systems (local activator, lateral, far-reaching inhibitor) with now regular patterns. Self-organization ability. Scaling. Only these systems, which expand Turing's approach in central points, allow a multitude of realistic space-time models in developmental biology. |
| 1976 | Dennis Summerbell (with Lewis Wolpert) | Progression zone model. Temporal effect of morphogen. |
| 1975/81 | Cheryll Tickle | ZPA transplants in different anterior positions; Experiments in support of the morphogen gradient model; Polarizing signals are dose-dependent. |
| 1982 | C. Tickle, B. Alberts, L. Wolpert, J. Lee; at the same time also D. Summerbell | Retinoic acid identified as the first morphogen capable of inducing a new polarizing region. |
| 1982/83 | Hans Meinhardt | Mathematical models of biological pattern formation. The first concrete biological examples based on Turing's theoretical work. Proximo-distal limb patterning model (Bootstrap model), comparable to Tabin and Wolpert 2007. |
| 1989 | P. Dolle, JC Izpisua-Belmonte, H. Falkenstein, A. Renucci, D. Duboule | Multiple use of homeotic structures in vertebrates, including in the extremity. |
| 1991 | JC Izpisua-Belmonte, D. Duboule, C. Tickle, L. Wolpert | Duboule / Tickle / Wolpert homeotic structures in the wing formation of birds. |
| 1993 | R.D: Riddle, RL Johnson, E. Laufer, Clifford J. Tabin | Sonic Hedgehog is identified as the ZPA's morphogen. |
| 1993 | Lee Niswander, C. Tickle, A. Vogel, I. Booth, GR Martin | The AER is the critical signal source that controls proximo-distal growth via the production of fibroblast growth factors (FGFs). |
| 1997 | J. Yang et al. | Studies on SHH in dose, time and distance effects. |
| 2001 | Zeng et al. | Direct evidence that SHH can diffuse long-range through the antero-posterior bud. |
| 2001 | Gritli-Linde et al. | Direct evidence that SHH can have a short-range and gradually far-reaching effect. |
| 2002 | Ying Litingtung et al. | Shh and Gli3 are dispensable, but regulate the number and identity of the toes (Fig. 17); Autopod without Shh and Gli3 expression is polydactyl without identities of the toes. |
| 2002 | AT Dudley, MA Ros, CJ Tabin | Early specification model as a counter model to the progressive zone model. |
| 2003/08 | Laura A. Lettice, Robert Hill et al. | ZRS as a cis regulator element for Shh; Discovery of several point mutations in the CRS that induce preaxial polydactyly in humans, mice and cats. |
| 2004 | BD Harp, PJ Scherz, S. Nissim, AP McMahon, CJ Tabin | Temporal effect of SHH in addition to the previous dose-dependency. Both dose and time are assumed to be the mechanisms of action for Shh. In detail, the following results for the different identities of the mouse fingers: The 5th and 4th fingers depend on the Shh expression duration; the 3rd finger depends on the Shh expression duration and the Shh concentration; the 2nd finger is only dependent on the Shh concentration and the 1st finger is Shh-independent (Fig. 15). These results are consistent with the model of stepped gene activation under a morphogenetic gradient influence by Meinhardt. |
| 2005 | Stuart A. Newman and Gerd B. Müller | A core set of cellular and molecular processes is responsible for self-organization in limb patterning. Position information is not required for cells. |
| 2005 | HG Hentschel, T. Glimm, JA Glazier, SA Newman | The Turing-based simulation model by Hentschel et al. Became the epigenetic-mathematical template for a number of models in the years that followed Stuart A. Newman |
| 2006 | S. Nissim, P. Allard, BD Harp, CJ Tabin | Determination of a new Tbx organizer region in the bud; it suppresses the anterior Shh expression. |
| 2006 | Takashi Miura et al. | Mixed-mode pattern in the double-foot mutant; Turing simulation of a growing bud shows different bifurcation points and finger widths. |
| 2006 | Cheryll Tickle | Cell-cell signals in mesenchymal toe spaces are responsible for the identity of the toes before apoptosis occurs. |
| 2007 | Clifford J. Tabin, Lewis Wolpert | Revision of the progression zone and early specification model. New view of the formation of the proximo-distal axis ( differentiation front model ) with regulatory interactions that control the temporary-dynamic expression of AP2, Meis1, Hoxa11, and Hoxa13. |
| 2008/09 | Johannes F. Knabe and others and Joachimczak / Wróbel. | French flag simulations: Computer-based morphogen gradients with individual cell differentiation in 2D and 3D. |
| 2009 | Matthew Towers, Ruth Mahood, Yili Yin Cheryll Tickle | Integration of growth and digit specification when patterning the chicken wing. |
| 2009 | Hans Meinhardt | Only a multi-level pattern leads to more exact borders and positions in the pattern formation. |
| 2009 | Jean-Dénis Bénazet, Rolf Zeller et al. | Self-regulating system of interacting feedback loops controls the pattern formation of the mouse extremity. |
| 2010 | J. Zhu, YT Zhang, MS Alber, SA Newman | Turing based, computer regulation network reproduces important properties of vertebrate extremity development and evolution. |
| 2011 | KL Cooper, MA Ros, CJ Tabin | Proximo-distal patterning: cells cannot interpret time. Instead, there is a dynamic balance between the proximal retinoic acid and the distal FGFs, which controls the proximo-distal segments in the chick. Opposite thesis to the progression zone model. |
| 2011 | A. Roselló-Diez, MA Ros, M. Torres | Diffusing signals, not autonomous mechanisms, determine the main subdivision of the proximo-distal axis. |
| 2012 | A. Badugu, D. Iber, et al. | BMP-receptor interaction in an integrated, systemic model with for the first time realistic 2D bud geometry |
| 2012 | Sheth et al. | Hox genes regulate the spacing and number of fingers, proven empirically and simulated in the Turing model. |
| 2011/06/13 | T. Montavon et al., B. Tarchini et al., G. Aundry et al. | Uncovering the mechanisms of colinearity in vertebrate extremity development in the mouse. In fact, the axial organization of our legs and arms corresponds to the linear organization of regulatory chromatin domains. The transition between these chromosomal domains corresponds to the wrist, that is, the transition between the old (arms and legs) and the new part (hands and feet) of our limbs. |
| 2014/08/1 | Raspopovic et al. | BSW-Turing model of the autopod based on the opposing, wave-like interaction of BMP and WNT in the spaces between the toes and SOX9 in the condensing toe elements. |
Other web links
- Limb Development (UNSW Embryology) Limb Development (UNSW Embryology)
- EMAP mouse atlas EMAP mouse atlas
- Caltech Mouseatlas Caltech Mouseatlas
- How do patterns develop in nature? How do digits develop on a limb? ~ Hox genes are the answer. How do patterns develop in nature? How do digits develop on a limb? ~ Hox genes are the answer.
See also
Individual evidence
- ^ A b c d e Matthew Towers, Cheryll Tickle: Growing models of vertebrate limb development. In: Development. 136, 2009, pp. 179-190.
- ↑ a b c Michael Kühl, Susanne Gessert: Developmental Biology . UTB basics, 2009.
- ↑ Lee Niswander: Pattern Information: Old Models out of a Limb. In: Nature Reviews Genetics . 4, February 2003, pp. 133-143.
- ↑ N. Soshnikova, D. Zechner, J. Huelsken, Y. Mishina, RR Behringer, MM Taketo, EB Crenshaw, W. Birchmeier: Genetic interaction between Wnt / beta-catenin and BMP receptor signaling during formation of the AER and the dorsal -ventral axis in the limb. In: Genes Dev. 17 (16), Aug 15, 2003, pp. 1963-1968.
- ↑ a b P. Dolle, JC Izpisua-Belmonte, H. Falkenstein, A. Renucci, D. Duboule: Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. In: Nature. 342, Dec 14, 1989, p. 767.
- ↑ P. Dolle, JC Izpisua-Belmonte, JM Brown, C. Tickle, D. Duboule: HOX-4 genes and the morphogenesis of mammalian genitalia. In: Genes Dev. 5, Oct 1991, p. 1767.
- ↑ a b J. C. Izpisua-Belmonte, C. Tickle, P. Dolle, L. Wolpert, D. Duboule: Expression of the homeobox Hox-4 genes and the specification of position in chick wing development. In: Nature. 350, Apr 18, 1991, p. 585.
- ↑ D. Duboule: Temporal colinearity and the phylotypic progression: a basis for the stability of a vertebrate building plan and the evolution of morphologies through heterochrony. In: Dev Suppl. 135, 1994.
- ↑ D. Duboule: The vertebrate limb: a model system to study the Hox / HOM gene network during development and evolution. In: Bioessays. 14, Jun, 1992, p. 375.
- ↑ P. Dolle et al .: Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. In: Cell. 75, Nov 5, 1993, p. 431.
- ^ F. Spitz, F. Gonzalez, D. Duboule: A global control region defines a chromosomal regulatory landscape containing the HoxD cluster. In: Cell. 113, May 2, 2003, p. 405.
- ↑ a b c T. Montavon et al: A regulatory archipelago controls Hox genes transcription in digits. In: Cell. 147, Nov 23, 2011, p. 1132.
- ^ A b B. Tarchini, D. Duboule: Control of Hoxd genes' collinearity during early limb development. In: Dev Cell. Jan 10, 2006, p. 93.
- ↑ a b c d G. Andrey et al .: A switch between topological domains underlies HoxD genes collinearity in mouse limbs. In: Science. 340, Jun 7, 2013, p. 1234167.
- ^ A b c Adam H. Rabinowitz, Steven A. Vokes: Interaction of the transcriptional networks regulating limb morphogenesis. In: Developmental Biology. 268, 2012, pp. 165-180.
- ↑ a b L. Wolpert: Positional information and the spatial pattern of cellular differentiation. In: Journal of Theoretical Biology. Vol. 25, Issue 1, Oct. 1969, pp. 1-47.
- ↑ M. Varjosalo, J. Taipale: Hedgehog: functions and mechanisms. In: Genes and Development. 2008, p. 2462.
- ^ Aimée Zuniga, Antonella Galli: Limb Pattern Formation. Upstream and Downstream of Shh Signaling. In: Carolyn E. Fisher, Sarah EM Howie (Eds.): Shh and Gli Signaling and Development. Landes Bioscience and Springer Science and Business Media, New York 2006, ISBN 1-4419-2294-6 .
- ↑ a b C. Tickle: Making digit patterns in the vertebrate limb. In: Nature Reviews Molecular Cell Biology . January 7, 2006, pp. 45-53.
- ^ A b c Alfred Gierer, Hans Meinhardt: A Theory of Biological Pattern Formation. In: Cybernetics. 12, 1972, pp. 30-39.
- ↑ a b c d e L. Wolpert: Positional Information and patterning revisited. In: Journal of Theoretical Biology. 269, 2011, pp. 359-365.
- ^ A b c Michael K. Richardson: Diffusible gradients are out - an interview with Lewis Wolpert. Interview. In: Journal of Developmental Biology. 53 (5-6), 2009, pp. 659-662.
- ↑ a b M. Kerszberg, L. Wolpert: Specifying Positional Information in the Embryo: Looking Beyond Morphogens. In: Cell. 130, July 27, 2007, pp. 205-209.
- ↑ a b D. Summerbell: A descriptive study of the rate of elongation and differentiation of the skeleton of the developing chick wing. In: Journal of embryology and experimental morphology. 35, 1976, pp. 241-260.
- ↑ a b Kimberly L. Cooper, Jimmy Kuang-Hsien Hu, Derk ten Berge, Marian Fernandez-Teran, Maria A. Ros, Clifford J. Tabin : Initiation of Proximal-Distal Patterning in the Vertebrate Limb by Signals and Growth. In: Science Magazine. 332, 6033, May 27, 2011, pp. 1083-1086.
- ↑ a b A. Roselló-Díez, MA Ros, M. Torres: Diffusible Signals, Not Auto-nomous Mechanisms, Determine the Main Proximo-distal Limb Subdivision. In: Science. Vol. 332, no. 6033, May 27, 2011, pp. 1086-1088.
- ^ A b Hans Meinhardt: A bootstrap model for the proximodistal pattern formation in vertebrate limbs. In: Journal of Embryological Experimental Morphology. 76, 1983, pp. 139-146.
- ↑ a b c d e Hans Meinhardt: Models for the Generation and Interpretation of Gradients. In: Cold Springs Harbor Perspectives in Biology. 1 (4), Oct 2009, p. A001362.
- ↑ a b B. D. Harfe, PJ Scherz, S. Nissim, H. Tian, AP McMahon, CJ Tabin : Evidence for an Expansion-Based Temporal Shh Gradient in Specifying Vertebrate Digit Identities. In: Cell. Volume 118, Issue 4, August 20, 2004, pp. 517-528.
- ↑ a b c C. Tabin, L. Wolpert: Rethinking the proximo-distal axis of the vertebrate limb in the molecular era. In: Genes & Dev. 21, 2007, pp. 1433-1442.
- ↑ a b c Jean-Denis Bénazet, Mirko Bischofberger, Eva Tiecke, Alexandre Gonçalves, James F. Martin, Aimée Zuniga, Felix Naef, Rolf Zeller: A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. In: Science. 323, 5917, 2009, pp. 1050-1053.
- ↑ a b c J. D. Bénazet, R. Zeller: Vertebrate Limb Development Moving from Classical Morphogen Gradient to an Integrated 4-Dimensional Pattern System. 2009.
- ↑ a b c d e Amarendra Badugu, Conradin Kraemer, Philipp Germann, Denis Menshykau, Dagmar Iber: Digit patterning during limb development as a result of the BMP-receptor interaction. In: Scientific Reports. 2, 991, 2012. doi: 10.1038 / srep00991
- ↑ a b c d e Y. Litingtung, RD Dahn, Y. Li, JF Fallon, C. Chiang: Shh and Gli3 are dispensable for limb skeleton formation but regulate digit number and identity. In: Nature. Volume 418, 2002, pp. 979-983.
- ^ Stuart A. Newman: Form and function remixed: developmental physiology in the evolution of vertebrate body plans. In: The Journal of Physiology. 592, 2014, pp. 2403-2412, doi: 10.1113 / jphysiol.2014.271437 .
- ↑ a b A. M. Turing: The chemical basis of morphogenesis. In: Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. Vol. 237, no. 641, Aug 14, 1952, pp. 37-72.
- ^ SA Newman, HL Frisch: Dynamics of Skeletal Pattern Formation in Developing Chick Limb. 1979.
- ^ GB Müller, GP Wagner: Novelty in Evolution: Restructuring the Concept. In: Annual Revue of Ecology, Evolution and Systematics. 22, 1991, pp. 229-256.
- ↑ a b c d e J. Zhu, Y.-T. Zhang, MS Alber, SA Newman: Bare Bones Patterning Formation: A Core Regulatory Network in Varying Geometrics Reproduces Major Feastures of Vertebrate Limb Development and Evolution. Online 2010.
- ↑ a b c d e H. G. Hentschel, T. Glimm, JA Glazier, SA Newman: Dynamical mechanisms for skeletal pattern formation in the vertebrate limb. In: Proceedings of the Royal Society B Biological Sciences. Volume 271, 2005, pp. 1713-1722.
- ↑ a b c d e f S. A. Newman, GB Müller: Origination and Innovation in the Vertebrate Limb Skeleton: An Epigenetic Perspective. In: Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2005, pp. 593-609.
- ^ GB Müller: Epigenetic Innovation. In: M. Pigliucci, GB Müller (Ed.): Evolution - The Extended Synthesis. MIT Press, 2010, pp. 307-332.
- ↑ a b c R. Chaturvedi, C. Huang, B. Kazmierczak, R. Schneider, T. Schneider, JA Izaguirre, T. Glimm, HGE Hentschel, SA Newman, JA Glazier, M. Alber: On Multiscale Approaches to Three- Dimensional Modeling of Morphogenesis. In: JR Soc Interface. June 22, 2005, pp. 237-253.
- ↑ K. Tamura, S. Yonei-Tamura, T. Yano, H. Yokoyama, H. Ide: The autopod: Its formation during limb development. In: Development, Growth & Differentiation. 50, 2008, pp. 177-187.
- ↑ a b c d Rushikesh Sheth, Luciano Marcon, M. Félix Bastida, Marisa Junco, Laura Quintana, Randall Dahn, Marie Kmita, James Sharpe, Maria A. Ros: Hox Genes Regulate Digit Patterning by Controlling the Wavelength of a Turing-Type Mechanism. In: Science. Vol. 338 no. 6113, December 14, 2012, pp. 1476–1480.
- ↑ a b c d e J. Raspopovic, L. Marcon, L. Russo, J. Sharpe: Digit patterning is controlled by a BMP-Sox9-Wnt Turing network modulated by morphogen gradients. In: Science. Vol. 345, Iss. 6196, August 2014, pp. 566-570.
- ↑ a b c D. Iber, R. Zeller: Making sense — data-based simulations of vertebrate limb development. In: Current Opinion in Genetics & Development. 22, 2012, pp. 1-8.
- ^ A b P. K. Maini, TE Woolley, RE Baker, EA Gaffney, SS Lee: Turing's model for biological pattern formation and the robustness problem. In: Interface focus. Volume 2, number 4, August 2012, pp. 487-496, doi: 10.1098 / rsfs.2011.0113 . PMID 23919129 , PMC 3363041 (free full text).
- ↑ a b c C. Tickle, H. Barker: The Sonic hedgehog gradient in the developing limb. In: Wiley interdisciplinary reviews. Developmental biology. Volume 2, number 2, 2013 Mar-Apr, pp. 275–290, doi: 10.1002 / wdev.70 . PMID 24009037 .
- ↑ a b T. Miura, K. Shiota, G. Morriss-Kay, PK Maini: Mixed-mode pattern in Doublefoot mutant mouse limb – Turing reaction-diffusion model on a growing domain during limb development. In: Journal of theoretical biology. Volume 240, Number 4, June 2006, pp. 562-573, doi: 10.1016 / j.jtbi.2005.10.016 . PMID 16364368 .
- ↑ A. Dekanty, M. Milán: The interplay between morphogens and tissue growth. In: EMBO reports. Volume 12, number 10, September 2011, pp. 1003-1010, doi: 10.1038 / embor.2011.172 . PMID 21886183 , PMC 3185346 (free full text) (review).
- ↑ a b K. Lai, MJ Robertson, DV Schaffer: The sonic hedgehog signaling system as a bistable genetic switch. In: Biophysical Journal. Volume 86, Number 5, May 2004, pp. 2748-2757, doi: 10.1016 / S0006-3495 (04) 74328-3 . PMID 15111393 , PMC 1304145 (free full text).
- ↑ Hannah H. Chang, Martin Hemberg, Mauricio Barahona, Donald E. Ingber, Sui Huang: Transcriptome-wide noise controls lineage choice in mammalian progenitor cells. In: Nature. Volume 453, number 7194, May 2008, pp. 544-547, doi: 10.1038 / nature06965 . PMID 18497826 .
- ^ NA Campbell, JB Reece: Biology. 6th, revised edition. Pearson Studies, 2006.
- ↑ a b c L. A. Lettice, AE Hill, PS Devenney, RE Hill: Point mutations in a distant sonic hedgehog cis-regulator generate a variable regulatory output responsible for preaxial polydactyly. In: Human Molecular Genetics . Volume 17, Number 7, April 2008, pp. 978-985, doi: 10.1093 / hmg / ddm370 . PMID 18156157 .
- ^ A b C. J. Tabin, AP McMahon: Developmental biology. Grasping limb patterning. In: Science. Volume 321, Number 5887, July 2008, pp. 350–352, doi: 10.1126 / science.1162474 . PMID 18635784 .
- ^ A. Wagner: The Origins of Evolutionary Innovations: A Theory of Transformative Change in Living Systems. 2011.
- ↑ Frietson Galis, Jacques JM van Alphen, Johan AJ Metz: Why five fingers? Evolutionary constraints on digit numbers. In: Trends in Ecology & Evolution. 16, 2001, p. 637, doi: 10.1016 / S0169-5347 (01) 02289-3 .
- ↑ KE Sears, RR Behringer, JJ Rasweiler, LA Niswander: Development of bat flight: morphologic and molecular evolution of bat wing digits. In: Proceedings of the National Academy of Sciences . Volume 103, Number 17, April 2006, pp. 6581-6586, doi: 10.1073 / pnas.0509716103 . PMID 16618938 , PMC 1458926 (free full text).
- ↑ K. Dahte: Molecular causes of isolated hand malformations using the example of the BMP signal path and SHH. Habilitation thesis . 2009.
- ↑ Cristina-Maria Crucial, Christof Niehrs: Secreted and Transmembrane Wnt Inhibitors and Activators. In: Cold Spring Harbors Perspective in Biology. 2012.
- ↑ a b c S. Nissim, P. Allard, A. Bandyopadhyay, BD Harfe, CJ Tabin: Characterization of a novel ectodermal signaling center regulating Tbx2 and Shh in the vertebrate limb. In: Developmental biology. Volume 304, Number 1, April 2007, pp. 9-21, doi: 10.1016 / j.ydbio.2006.12.010 . PMID 17300775 , PMC 1868507 (free full text).
- ↑ SJ Driess: Point mutation analyzes in GLI3-associated diseases: Greig cephalopolysyndactyly syndrome, Pallister-Hall syndrome and isolated polydactyls. Dissertation. 2005.
- ^ GP Wagner, HCE Larsson: Fins and Limbs in the Study of Evolutionary Novelties. In: Brian K Hall: Fins into Limbs, Evolution, Development and Transformation. University of Chicago Press, Chicago / London 2007.
- ↑ C. Fromental-Ramain, X. Warot, N. Messadecq, M. LeMeur, P. Dollé, P. Chambon: Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. In: Development. Vol. 122, number 10, October 1996, pp. 2997-3011. PMID 8898214 .
- ↑ CJ Tabin: Why we have (only) five fingers per hand: hox genes and the evolution of paired limbs. In: Development. Volume 116, Number 2, October 1992, pp. 289-296. PMID 1363084 (Review).
- ↑ G. Vogel: Developmental biology. Turing pattern fingered for digit formation. In: Science. Volume 338, number 6113, December 2012, p. 1406, doi: 10.1126 / science.338.6113.1406 . PMID 23239707 .
- ^ C. Tickle: The Early History of the Polarizing Region: from Classical Embryology to Molecular Biology. In: International Journal of Development Biology. Volume 46, 2002, pp. 847-252.
- ^ JL Galloway, CJ Tabin: Classic limb patterning models and the work of Dennis Summerbell. In: Development. 135 (16), Aug 2008, pp. 2683-2687.
- ↑ a b L. A. Lettice, SJH Heaney, LA Purdie, L. Li, P. de Beer, BA Oostra, D. Goode, G. Elgar, RH Hill, E. de Graaff: A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. In: Human Molecular Genetics. Vol. 12, No. 14, 2003, pp. 1725-1735.
- ↑ B. Boehm, M. Rautschka, L. Quintana, J. Raspopovic, J. Ziga, J. Sharpe: A landmark-free mrphogenetic staging system for the mouse limb bud. In: Development. 138, 2011, pp. 1227-1234.
- ^ SA Newman, R. Bhat: Activator-Inhibitor Dynamics of Vertebrate Limb. In: Birth Defects Research (Part C). 81, 2007, pp. 305-319.
- ↑ Vicki L. Church, Philippa Francis-West: Wnt signaling during limb development. In: International Journal of Developmental Biology. 46, 2002, pp. 927-936.
- ↑ KE Kmetzsch: Molecular and Cellular Mechanisms whereby the apicalectordermal Ridge (AER), via WNT5A, mediates directional migration of the adjacent Mesenchyme during vertebrate Limb Development. Master thesis. 2009.
- ↑ Jean-Claude Beetschen: Louis Sébastian Marie de Tredern de Lézérec (1780-18?), A forgotten pioneer of chic embryology. In: Int. J. Dev. Biol. 39, 1995, pp. 299-308.
- ^ W. Saunders, MT Gasseling: The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. In: Journal of Experimental Zoology. 108 (3), Aug 1948, pp. 363-403.
- ^ W. Saunders, MT Gasseling: Ectodermal-mesenchymal interactions in the origin of limb symmetry. 1968. In: Wilhelm Roux's archives of developmental biology. Volume 182, Issue 3, 1977, pp. 213-225.
- ↑ C. Tickle: Positional signaling and specification of digits in chick limb morphogenesis. In: Nature. 254, 1975, pp. 199-202.
- ↑ C. Tickle, B. Alberts, L. Wolpert, J. Lee: Local application of retinoic acid to the limb bond mimics the action of the polarizing region. In: Nature. 1982.
- ↑ D. Summerbell: The effect of local application of retinoic acid to the anterior margin of the developing chick limb. In: Journal of Embryological Experimental Morphology. 78, 1983, pp. 269-289.
- ^ H. Meinhardt: Models of Biological Pattern Formation. Academic Press, London 1982.
- ^ RD Riddle, RL Johnson, E. Laufer, Clifford J. Tabin: Sonic hedgehog mediates the polarizing activity of the ZPA. In: Cell. Vol. 75, Issue 7, December 31, 1993, pp. 1401-1416.
- ↑ L. Niswander, C. Tickle, A. Vogel, I. Booth, GR Martin : FGF-4 replaces the apical ectodermal ridge and directs outgrowth and patterning of the limb. In: Cell. 75, 1993, pp. 579-587.
- ↑ J. Yang, G. Drossopoulou, PT Chuang, D. Duprez, E. Marti, D. Bumcrot, N. Vargesson, J. Clarke, L. Niswander, A. McMahon, C. Tickle: Relationship between dose, distance and time in Sonic Hedegehog-mediated regulation of anterior-posterior polarity in the limb. In: Development. Volume 124, 1997, pp. 4393-4407.
- ↑ X. Zeng, JA Goetz, LA Suber, WJ Scott Jr, CM Schreiner, DJ Robbins: A freely diffusible form of Sonic Hedgehog mediates long range signaling. In: Nature. 411, 2001, pp. 716-720.
- ^ A. Gritli-Linde, P. Lewis, AP McMahon, A. Linde: The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. 2001.
- ^ AT Dudley, MA Ros, CJ Tabin: A re-examination of proximo-distal patterning during vertebrate limb development. In: Nature. 418, 2002, pp. 539-544.
- ↑ JF Knabe, MJ Schilstra, C. Nehaniv: Evolution and Morphogenesis of Differenciated Multicellular Organisms: Autonomously Generated Diffusion Gradients for Positional Information. In: Artificial Life. 2008, pp. 321-328, XI.
- ↑ M. Joachimczak, B. Wróbel: Evolution of the morphology and patterning of artificial embryos: scaling the tricolor problem to the third dimension. In: LNCS. vol. 5777, 2009, pp. 33-41.
- ↑ M. Towers, R. Mahood, Y. Yin, C. Tickle: Integration of growth and specification in chick wing digit-patterning. In: Nature. 789, 2009, pp. 882-886.