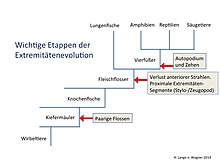
Extremity evolution
When evolution of the extremities , the conversion of the pair will fins of bony fish (Osteichthyes) at the front and rear limbs of terrestrial vertebrates (Tetrapoda) in the course of phylogeny referred to as well as the subsequent evolutionary change in form of the limbs to the shore of vertebrates.
introduction
The evolution of body appendages to achieve mobility was an important innovation in the Ordovician . This innovation first appeared in jawless vertebrates, each with a narrow, long, striped fin on the back and abdomen. In others, paired pectoral fins formed directly behind the head. The appearance of paired fins is a synapomorphism , i.e. H. a new property of the jaws (Gnathostomata). Comparative developmental analyzes of genes responsible for pattern formation in the tetrapod extremity show that they are arranged in paired fish fins. Regardless of a deep homology of the basic structures and the underlying developmental processes and genetics, questions remain unanswered today regarding the complicated phenotypic reconstruction of the distal skeletal fish fin parts to distal elements of the tetrapod extremity (hand / foot). Certain skeletal elements had to disappear evolutionarily, while others, distal, were newly added. The transformation from fins to tetrapod extremities was one of the most significant macroevolutionary variations in vertebrate history.
Designs
The paired fins of fish and extremities of tetrapods are homologous skeletal elements insofar as they attach to the shoulder and pelvic girdles in both and the extremities have evolved from the paired fins. However, they differed in bone structure and in embryonic development, so that an evolutionary transition from the individual elements is difficult to explain.
Paired ray fins

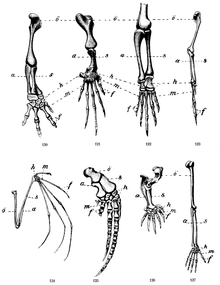
In bony fish (Osteichthyes) and cartilaginous fish (Chondrichthyes), the extremities of the tetrapods correspond to the pectoral and pelvic fins , which are called ray fins . Inside the base of the fin there are usually at least four proximal bones close to the trunk (basals) in the antero-posterior direction, in contrast to one bone (zeugopod) in tetrapods. In the more highly developed radiation fins (Actinopterygii), the fin ray carriers (the four basalia or radialia) attach directly to the primary shoulder girdle (scapula, coracoid). They carry the fin rays , the Lepidotrichia, which have arisen from scales. The predominant part of the ray fins consists of the bony fin rays (Radii, Sg. Radius) and the webbing (patagium), which is spanned by the radii. In all bony fish, the muscles for the fin movement are only attached to the base of the fin. Correspondingly, the entire base of the fin, ie the skeleton of the fin base and muscles, is rather inconspicuous in radiation fins. Rays are not homologous to fingers and toes. The bones of lungfish and coelacanth are more similar to the shape of the tetrapod extremity.
Tetrapod extremity
The tetrapod extremity consists of a long bone (stylopodium), the humerus ( humerus ) or thigh bone (femur), which is connected to the shoulder or pelvis via a joint. This is followed by Zeugopodium connects two parallel long bones, the arm Elle (ulna) and spoke (radius) connected by the elbow joint , corresponding at the foot of the tibia (tibia) and fibula (fibula) with the overlying bone joined by the knee joint . In the distal direction, the car platform (hand / foot) follows, which are initially arm the carpal bone (carpal bone) or on the leg the tarsal bones (tarsal). The fingers on the arm and the toes on the foot are tied distally to this. The number of distal elements on an extremity is variable. As the "basic type" it is 5 and can be reduced to one toe. It does not have to be the same on the front and rear extremities.
Transformation fin - extremity
Anatomical comparisons


Richard Owen described in 1849 in On the Nature of Limbs ("On the nature of the limbs") the systematics of vertebrate extremities. He discovered the regular occurrence of a large bone connected to the shoulder or pelvis via a joint, connected to it via a joint in the proximo-distal direction, two further parallel long bones, then a series of small bones and finally the hand or toe bones of up to 5 distal elements. Owen recognized the corresponding bones as homologous in humans, other mammals, and birds. In addition, he saw the pectoral and pelvic fins of fish as homologous to the extremities of the tetrapods.
The homology of fish fins with tetrapod extremities has long remained a mystery, since no bone relationship could be established. At the end of the 19th century, Eusthenopteron , a 380-million-year-old coelacanth fossil from the Canadian province of Quebec, was considered a candidate for an amphibian precursor because it had been identified as a single bone on the shoulder girdle, which was interpreted as a homologous part of the upper arm. Ichthyostega , discovered on the east coast of Greenland in the early 1930s, showed complete limbs for the first time, including fingers and toes. Its vertebrae were amphibian-like. Acanthostega , also found in the Devonian and in East Greenland, also has complete limbs, but also 8 radially arranged toes on the front and rear extremities, which form a paddle-like fin. Its fore and hind limbs were built so that the bones could not support the heavy body on the land. Acanthostega also lacked the joints on the elbows and knees for sufficient locomotion on land. The limbs of the terrestrial vertebrates are therefore not an evolutionary change in the course of the shore leave, but were already developed in the water, especially since there must have been predecessor stages between Ichthyostega or Acanthostega and previously living fish. In 1995 a 365 million year old fish extremity ( Sauripterus ) was found in Philadelphia, USA . The associated body is missing. The animal had a single bone on the trunk and eight bone rods on the edge of the fins. These have been described as toe precursors.
Tiktaalik , an Amhibian-like, 375 million year old fossil found by Neil Shubin and colleagues on Ellesmere Island in 2004, is most similar to the extremity principle described by Owen. The animal has the shoulder, elbow and wrist, consisting of the same upper arm, forearm and wrist bones as in humans. It could lean on this limb, much like recent mudskippers . To do this, the elbow could bend, supported by large chest muscles. Unlike humans, elbows and knees point in the same direction. Pederpes from the early Carboniferous , about 30 million years after Tiktaalik, is seenas the first tetrapod with 5 forward-facing toes on the limbs suitable for walking on land.
Neither in Sauripterus nor Tiktaalik can the early transformation of a fin with rays into an extremity with toes be traced. There are no intermediate forms here. The transition is largely accompanied by the irreversible loss of skeletal elements and the simultaneous evolutionary innovation of other elements (autopodium). The result is that no homologous bones exist between the mouse extremity and the zebrafish pectoral fin.
Development comparisons
In tetrapod extremity, the mesenchyme of the bud condenses . Mesenchymal cells aggregate and then differentiate into cartilage cells (see extremity development ). The individual elements are first developed and then chondrogenised into cartilage. The development process is reversed in the ray fin of the bony fish. First the cartilage is formed and then the individual differentiation of the individual elements takes place.
The embryonic development of the extremities in chickens and mice is partly determined by an organizing region ZPA (zone of polarizing activity) on the posterior side of the bud. The protein Sonic hedgehog is released in the ZPA (see extremity development ). If this region is removed in the laboratory from the chicken embryo and implanted on the anterior side of another in addition to the existing ZPA, the toes are mirror-inverted. This experiment was carried out on the ray , a cartilaginous fish. It could be shown that the fin also has doubling of distal elements. This was also possible with the shark , also a cartilaginous fish. A relationship between genetic development processes in the fin of evolutionarily old fish and those in the limb bud of chickens and mice could be demonstrated. Comparisons could also be made with the expression of Alx4 , Pax9 , Hand2 and Gli3 in the pectoral fin of the cat shark and the limb bud of the mouse, which provide clues for the transition from fin to tetrapod extremity. Likewise, comparable (three-phase) expression patterns were discovered in the pectoral fin of the zebrafish for certain Hox genes , which are known from extremity development. In an experiment, Hox genes from zebrafish were expressed in the bud of the mouse and showed proximal expression patterns there. This demonstrated that these gene regulation mechanisms existed before the fin-extremity transformation. Increased efforts are now being made to analyze changes in cis-elements , which play a key role in gene regulation of the fin or extremity , instead of mutations in developmental genes .
Variability and remodeling of the tetrapod extremity
Robustness of the pentadactylene design
The tetrapod extremity is a robust anatomical shape. The five-fingered (pentadactyl) system occurs in mice and toads as well as in elephants, horses and whales. Since it cannot be assumed that the embryos of such different species are of the same size at the time of extremity development, the development mechanisms relevant for further training must be evolutionarily size-independent. At the same time, the extremity is evolutionarily characterized by a high adaptability, as can be seen from the different shapes in vertebrate species. Both properties seem intuitively contradictory. However, Andreas Wagner was able to show that robust forms with pronounced gene networks generally have better conditions for variation and innovation than less robust ones.
The pentadactyl upper limit of the hand (five fingers) has not yet been conclusively clarified. As two possible reasons, Jennifer A. Clack states that the extremity bud has a physically limited extent in embryonic development. This allows either a larger number of smaller toes or a smaller number of larger toes. The requirement to have to carry your body weight when walking speaks in favor of a smaller number of stronger toes. Secondly, five toes are considered to be the best and most stable alternative from an economic point of view for the arrangement on the carpal bones (hand) or tarsal bones (foot) in order to be able to exercise the required inward rotation ( pronation ) and outward rotation ( supination ).
Early tetrapods (e.g. Acanthostega ) were interpreted as polydactyl rather than seven or eighteen toe because of the similarity of the toes. Jennifer A. Clack is of the opinion that Acanthostega does not have two identical toes on any extremity and thus the number of toes is neither pentadactyl nor polydactyl. Vertebrate species that normally have more than five fingers or toes on one extremity are very rare. The giant panda and mole have deformed metacarpal bones that look like toes. Exceptions with selective human influence are certain polydactyl populations of cats ( Maine Coons ) and the Norwegian Lundehund . Galis cites development constraints as the reason for preventing a larger number of fingers. A study from 2015 shows the presence of a real sixth toe preaxial to the big toe (Pollux) on the hind foot of a clawed frog ( Xenopus tropiclais ). This is the first empirical evidence of standard six toes in a recent species of tetrapod.
Variability of the tetrapod design
The bird's wing has three toes. During development, the bud initially shows five systems , four of which chondrogenize in the condensation phase and only the three middle ones ossify . Toe systems could thus be preserved for millions of years. A second theory of toe reduction in bird wings and alligators , presented by Günter P. Wagner , is based on a homoiotic transformation (homeosis). It says here that toes 1, 2 and 3 grow at the embryonic positions of toes 2, 3 and 4 and assume their identity.
The extreme toe length of the bat's front extremity (wings) is at an early embryonic stage of development (day 16 after fertilization) and thus after the condensation and segmentation phase in the extremity bud is about the same length as that of the mouse on day 12.5. The lengthening of toes 3, 4 and 5 of the bat in particular from day 20 onwards could be associated with increased expression of the bone morphogenetic protein Bmb2. Bmp2 stimulates cartilage formation and differentiation and increases the toe length in embryonic development. The evolution of the bat is thus justified by changes in the development of length growth and the length differentiation of cartilage tissue in the front extremity.
See also
Extremity development (tetrapods)
Web links
- How limbs evolved from fins. Scitable by Nature Education February 17, 2014.
- Tohru Yano, Koji Tamura: The making of differences between fins and limbs. In: J Anat. 222 (1), Jan 2013, pp. 100–113, PMC 3552418 (free full text)
- Genetic clue to how limbs evolved from fins. BBC News, Science & Environment. January 24, 2014.
- Elizabeth Mitchell: Fish Fins Are Not Fingers That Failed. January 28, 2014.
- How did paired fins evolve? How did limbs evolve? Courses Washington Edu.
Individual evidence
- ↑ a b c d A. A. Abbasi: Evolution of vertebrate appendicular structures: Insight from genetic and palaeontological data. In: Dev Dyn. 240 (5), May 2011, pp. 1005-1016. doi: 10.1002 / dvdy.22572 .
- ↑ a b c d Günter P. Wagner: Homology, Genes and Evolutionary Innovation. Princeton University Press, 2014.
- ^ Peter J. Bowler: Find and Limbs and Fins into Limbs. The Historical Context 1840-1940. In: Brian K. Hall (Ed.): Fins into Limbs. Evolution, Development and Transformation. University of Chicago Press, 2007, ISBN 978-0-226-31336-8 , pp. 7-14.
- ↑ a b c Neil Shubin: The Fish Within. A journey through the 3.5 billion history of our body. S. Fischer 2008.
- ↑ Jennifer A. Clack: Gaining Ground. The Origin and Evolution of Tetrapods. 2nd Edition. Indiana University Press, 2012, ISBN 978-0-253-00537-3 , p. 173.
- ↑ Jennifer A. Clack: Gaining Ground. The Origin and Evolution of Tetrapods. Indiana University Press, 2012, p. 441.
- ^ EB Daeschler, NH, Shubin, KS Thomson, WW Amaral: A Devonian tetrapod from North America. In: Science. 265, Jul 1994, pp. 639-642.
- ↑ Jennifer A. Clack: Gaining Ground. The Origin and Evolution of Tetrapods. Indiana University Press, 2012, p. 273.
- ↑ a b A. Wagner: The Origins of Evolutionary Innovations: A Theory of Transformative Change in Living Systems. 2011.
- ^ W. Saunders, MT Gasseling: The proximo-distal sequence of origin of the parts of the chick wing and the role of the ectoderm. In: Journal of Experimental Zoology. 108 (3), Aug 1948, pp. 363-403.
- ↑ Center of Genomic Regulation. Key genetic event underlying fin-to-limb evolution . (August 18, 2015)
- ↑ Daegwon Ahn, Robert K. Ho: Tri-phasic expression of posterior Hox genes during development of pectoral fins in zebrafish: Implications for the evolution of vertebrate paired appendages. In: Developmental Biology. Vol. 322, Issue 1, October 1, 2008, pp. 220-233.
- ↑ Marcus C. Davis: The Deep Homology of the Autopod: Insights from Hox Gene Regulation. In: Integr. Comp. Biol. 2013. doi: 10.1093 / icb / ict029 .
- ↑ JM Woltering, D. Noordermeer, M. Leleu, D. Duboule : Conservation and Divergence of Regulatory Strategies at Hox Loci and the Origin of Tetrapod Digits. In: PLoS Biol. 12 (1), 2014, p. E1001773. doi: 10.1371 / journal.pbio.1001773
- ↑ Note: The tetrapod extremity developed from the flesh fin (Sarcopterygium) of the Sarcopterygii , which are only distantly related to the mouse. Their extremities thus have little to do with the actinopterygic fin (see Fig. "Important stages of extremity evolution"). Therefore, a comparison of the mouse extremity with the pectoral fin of the zebrafish (an actinopterygian) is only meaningful to a limited extent
- ↑ Jennifer A. Clack: Gaining Ground. The Origin and Evolution of Tetrapods. 2012, p. 253.
- ↑ Note: On the opposite position see: Joost M. Woltering, Axel Meyer : The phantoms of a high-seven - or - why do our thumbs stick out? In: Front Zool. 12, 2015, p. 23. doi: 10.1186 / s12983-015-0117-x . PMC 4570229 (free full text)
- ↑ Jennifer A. Clack: Gaining Ground. The Origin and Evolution of Tetrapods. Indiana University Press. 2012, pp. 136, 168.
- ↑ F. Galis, JJM Alphen: Why Five Fingers? Evolutionary Constraints on Digit Numbers. In: Trends in Ecology and Evolution. Volume 16, Number 11, November 1, 2001, pp. 637–646 (10)
- ↑ Shinichi Hayashi, Takuya Kobayashi, Tohru Yano, Namiko Kamiyama, Shiro Egawa, Ryohei Seki, Kazuki Takizawa, Masataka Okabe, Hitoshi Yokoyama, Koji Tamura: Evidence for an amphibian sixth digit. In: Zoological Letters. 1, 2015, p. 17. doi: 10.1186 / s40851-015-0019-y
- ↑ D. Capek, BD Metscher, GB Müller : Thumbs Down: a molecular-morphogenetic approach to avian digit homology. In: J. Exp. Zool. (Mol. Dev. Evol.). 322B, 2014, pp. 1-12.
- ^ Günter P. Wagner: The developmental evolution of avian digit homology: An update. In: Theory in Biosciences. 124, 2005, pp. 165-183.
- ^ Karen E. Sears, Richard R. Behringer, John J. Rasweiler, Lee A. Niswander: Development of bat flight: Morphologic and molecular evolution of bat wing digits. In: PNAS. vol. 103, no. 17, April 25, 2006, pp. 6581-6586.