Febuxostat
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
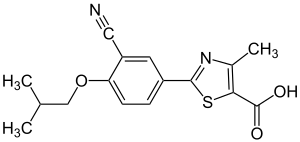
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Febuxostat | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 16 H 16 N 2 O 3 S | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 316.38 g · mol -1 | |||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| solubility |
0.2 mg l −1 in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Febuxostat ( trade name ADENURIC ® , sales Berlin-Chemie / Menarini ) is a drug that the treatment of chronic hyperuricaemia in conditions which already deposits of urate crystals have led (including a well-known from the medical history, or presence of , tophus and / or gouty arthritis ) is allowed.
pharmacology

|

|
|
| Purine | ||
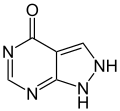
|
||
| Allopurinol | Febuxostat | |
Febuxostat belongs - together with allopurinol - to the group of active substances called uricostatics , but, as can be seen from the table above, it is a completely different substance and not a purine derivative like allopurinol.
Pharmacodynamics
As a selective inhibitor of xanthine oxidase , it leads to reduced uric acid formation through non-competitive inhibition of xanthine oxidase, which oxidizes hypoxanthine into xanthine and further into uric acid. In addition, the resulting increased hypoxanthine concentration inhibits purine synthesis . Febuxostat is a more selective and effective inhibitor of xanthine oxidase than allopurinol. Experimental results indicate that febuxostat inhibits both the oxidized and the reduced form of xanthine oxidase. In a therapeutic concentration, in contrast to allopurinol, febuxostat does not inhibit any other enzymes of the purine or pyrimidine metabolism (guanine deaminase, hypoxanthine-guanine phosphoribosyl transferase, orotate phosphoribosyl transferase, orotidine monophosphate decarboxylase or purine nucleoside phosphate).
Pharmacokinetics
- About 99.2% of febuxostat is bound to plasma proteins (primarily albumin).
- Febuxostat is eliminated both by the synthesis of the liver and the kidneys.
- The absorption of febuxostat is rapid and high (at least 84%).
- T max * = 1.0 - 1.5 h
- After single or repeated oral doses of febuxostat 80 and 120 mg once daily, the C max * is approximately 2.8-3.2 μg / ml and 5.0-5.3 μg / ml, respectively. The absolute bioavailability of the tablet formulation of febuxostat has not yet been investigated.
- The mean terminal elimination half-life ( t 1/2 ) of febuxostat is approximately 5 to 8 hours.
- The degradation takes place in the liver via the uridine diphosphate glucuronyl transferase (UDPGT) enzyme system and the cytochrome P450 (CYP) system. Oxidative metabolites are mainly formed by CYP1A1, CYP1A2, CYP2C8 or CYP2C9 and febuxostat glucuronide mainly by UGT1A1, 1A8 and 1A9.
- The elimination takes place in roughly equal parts both via the liver and the kidneys.
In the case of mild to moderate impairment of kidney function (creatinine clearance 30 - 80 ml / min), no dose adjustment is necessary. Neither age nor gender have a significant influence on the pharmacokinetics of febuxostat, so that no dose adjustment has to be made in patients over 65 years of age. After multiple doses of 80 mg febuxostat in subjects with mild or moderate hepatic impairment, the Cmax and AUC did not change significantly compared to subjects with normal hepatic function. No studies have been performed in patients with severe hepatic impairment.
Drug interactions
Febuxostat can be co-administered with colchicine (0.6 mg twice daily), with NSAIDs such as naproxen and indomethacin, hydrochlorothiazide, warfarin and desipramine (CYP2D6 substrate) without dose adjustment . Since azathioprine and 6-mercaptopurine are metabolized via xanthine oxidase and therefore severe side effects are possible from co-medication with febuxostat, simultaneous use is not recommended.
effectiveness
In a randomized study, Febuxostat lowered the uric acid level to normal values more reliably than allopurinol . The recurrence of symptomatic gout or the reduction in gout deposits were comparable.
unwanted effects
Liver dysfunction was observed as side effects of 3.5%. This rate has been observed in a comparable manner with allopurinol administration. Therapy had to be discontinued in around half of the cases with liver dysfunction. Less common side effects were diarrhea, nausea and allergic rash. An increased rate of cardiac events has not yet been confirmed. Stevens-Johnson syndrome, which is very rarely observed with allopurinol, did not occur in the approval studies with febuxostat. However, severe hypersensitivity reactions, including Stevens-Johnson syndrome, have rarely occurred after marketing authorization. An increase in patients with previous allergic reactions to allopurinol has been observed.
In the approval studies, more frequent cardiovascular events were noted with febuxostat than with allopurinol. This led to a corresponding warning in the German technical information. In November 2017, the US Food and Drug Administration ( FDA) also warned of an increased risk of cardiac death and announced an investigation. The manufacturer of febuxostat was obliged by the European Medicines Agency ( EMA ) and the FDA to investigate the cardiovascular safety of febuxostat in a prospective randomized study. This so-called CARES study included more than 6000 patients with gout and significant previous cardiovascular diseases. In comparison to treatment with allopurinol, it was found that both total mortality (7.8% vs. 6.4%) and mortality due to cardiovascular diseases (4.3% vs. 3.2%) were significantly higher with febuxostat was (HR: 1.22 and HR: 1.34, respectively). The pharmaceutical letter therefore warns that febuxostat should be used very cautiously, especially in patients with a high cardiovascular risk.
literature
- HR Schumacher, MA Becker, E. Lloyd, PA MacDonald, C. Lademacher: Febuxostat in the treatment of gout: 5-year findings of the FOCUS efficacy and safety study . In: Rheumatology . tape 48 , 2009, p. 188-194 , PMID 19141576 ( oxfordjournals.org ).
- K. Okamoto, BT Eger, T. Nishino, S. Kondo, EF Pai, T. Nishino: An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition . In: The Journal of Biological Chemistry . tape 278 , no. 3 , January 2003, p. 1848-1855 , PMID 12421831 ( jbc.org [PDF]).
- NL Edwards: Febuxostat: A new treatment for hyperuricemia in gout. In: Rheumatology , Vol. 48 No. 1 2009, pp. Ii15-ii19
- HR Schumacher, MA Becker, RL Wortmann, PA MacDonald, B. Hunt, J. Streit, C. Lademacher, N. Joseph-Ridge: Effects of Febuxostat versus Allopurinol and Placebo in Reducing Serum Urate in Subjects with Hyeruricemia and Gout: A 28 -Week, Phase III, Randomized, Double-Blind, Parallel-Group Trial. In: Arthritis Rheum . , 59, 2008, pp. 1540-1548.
- MA Becker, HR Schumacher, RL Wortmann, PA MacDonald, D. Eustace, WA Palo, J. Streit, N. Joseph-Ridge: Febuxostat compared with Allopurinol in Patients with Hyperuricemia and Gout. In: N Engl J Med . , 353, 2005, pp. 2450-2461.
- MA Becker, HR Schumacher, PA MacDonald, E. Lloyd, C. Lademacher: Clinical Efficacy and Safety of Successful Longterm Urate Lowering with Febuxostat or Allopurinol in Subjects with Gout. In: J Rheumatol . , 36, 2009, pp. 1273-1282.
- MA Becker, HR Schumacher, RL Espinoza, AF Wells, B. Hunt, PA MacDonald, E. Lloyd, C. Lademacher: The urat-lowering efficacy and safety of Febuxostat in the treatment of the hyeruricemia of Gout: The CONFIRMS trial . In: Arthritis Research & Therapy , 12, 2010, p. R63. doi: 10.1186 / ar2978
Web links
- Article about the drug. In: Deutsches Ärzteblatt
- Entries in the NIH study registry
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) for: Febuxostat
- Rote-Hand- Liefe on Febuxostat since May 2012
Individual evidence
- ^ A b The Merck Index : An Encyclopedia of Chemicals, Drugs, and Biologicals. 14th edition. Merck & Co., Whitehouse Station NJ 2006, ISBN 0-911910-00-X .
- ↑ K. Takács-Novák, M. Urac, P. Horváth, G. Völgyi, BD Anderson, A. Avdeef: Equilibrium solubility measurement of compounds with low dissolution rate by Higuchi's Facilitated Dissolution Method. A validation study. In: Eur. J. Pharm. Sci. , 106, 2017, pp. 133–144, doi: 10.1016 / j.ejps.2017.05.064 .
- ↑ There is not yet a harmonized classification for this substance . A labeling of 2- (3-Cyano-4- (2-methylpropoxy) phenyl) -4-methylthiazole-5-carboxylic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on, is reproduced from a self-classification by the distributor February 10, 2020.
- ↑ M. Hu, B. Tomlinson: Febuxostat in the management of hyperuricemia and chronic gout: a review . In: Therapeutics and Clinical Risk Management . tape 4 , no. 6 , December 2008, pp. 1209-1220 , PMID 19337428 , PMC 2643102 (free full text).
- ^ MA Becker, HR Schumacher, RL Wortmann u. a .: Febuxostat compared with allopurinol in patients with hyperuricemia and gout . In: The New England Journal of Medicine . tape 353 , no. December 23 , 2005, pp. 2450-2461 , doi : 10.1056 / NEJMoa050373 , PMID 16339094 .
- ^ MA Becker, HR Schumacher Jr, RL Wortmann, PA MacDonald, D. Eustace: Febuxostat compared with allopurinol in patients with hyperuricemia and gout. In: N Engl J Med. 353 (23), December 8, 2005, pp. 2450-2461. PMID 16339094
- ^ A b P. Christalla, K. Wittköpper, A. Al-Armouche: Febuxostat, a new drug for the treatment of gout. In: The cardiologist. January 2011, pp. 45-50.
- ↑ Rote-Hand-Brief Important information on the relationship between the risk of severe hypersensitivity reactions, including Stevens-Johnson syndrome and acute anaphylactic reactions / shock with Adenuric® (febuxostat) . May 21, 2012, akdae.de (PDF); Retrieved May 27, 2012.
- ↑ Uloric (febuxostat): Drug Safety Communication - FDA to Evaluate Increased Risk of Heart-related Death. In: Drug safety alerts for human medical products. US Food and Drug Administration, 2017, accessed April 26, 2018 .
- ^ William B. White, Kenneth G. Saag, Michael A. Becker, Jeffrey S. Borer, Philip B. Gorelick: Cardiovascular Safety of Febuxostat or Allopurinol in Patients with Gout . In: New England Journal of Medicine . tape 378 , no. 13 , p. 1200–1210 , doi : 10.1056 / nejmoa1710895 ( nejm.org [accessed April 6, 2018]).
- ↑ Febuxostat: increased cardiovascular risk compared to allopurinol in adults with gout. In: The medicament letter . WD Ludwig, J. Schuler, 2018, accessed April 26, 2018 .