Flucythrinate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
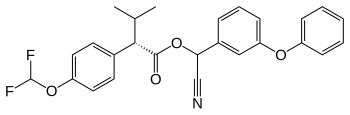
|
||||||||||||||||
| Structural formula without complete stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Flucythrinate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 26 H 23 F 2 NO 4 | |||||||||||||||
| Brief description |
colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 451.46 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.189 g cm −3 (22 ° C) |
|||||||||||||||
| boiling point |
108 ° C at 0.47 hPa |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.541 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Flucythrinate is a mixture of isomeric chemical compounds from the group of pyrethroids and an analogue of fenvalerate .
Extraction and presentation
Flucythrinate can be obtained by a multi-stage reaction of p -cresol with chlorodifluoromethane , bromine , sodium cyanide , 2-chloropropane , sodium hydroxide , potassium hydroxide and thionyl chloride .
properties
Flucythrinate is a colorless liquid. The technical product is a brown liquid with a faint odor. The compound is stable but hydrolyzes rapidly under alkaline conditions.
use
Flucythrinate is used as an insecticide and acaricide . The effect is based on influencing the sodium channels .
Admission
Flucythrinate was approved in the GDR between 1984 and 1992.
In 2002, the EU Commission decided not to include flucythrinate in the list of permitted active ingredients in pesticides according to Annex I of Directive 91/414 / EEC.
In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c d e f g h i Flucythrinate data sheet from Sigma-Aldrich , accessed on May 22, 2017 ( PDF ).
- ↑ a b c Entry on flucythrinate in the Pesticide Properties DataBase (PPDB) of the University of Hertfordshire , accessed August 1, 2013.
- ↑ a b Entry on Flucythrinate in the Hazardous Substances Data Bank , accessed November 27, 2012.
- ↑ a b Terence Robert Roberts, Terence Robert Roberts DH Hutson: Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides . Royal Society of Chemistry, 1999, ISBN 0-85404-499-X , pp. 667 ( limited preview in Google Book search).
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 950 ( limited preview in Google Book Search).
- ↑ Peter Brandt: Reports on Plant Protection Products 2009: Active Ingredients in Plant Protection Products ; Approval history and regulations of the Plant Protection Application Ordinance . Springer DE, 2010, ISBN 3-0348-0028-2 , pp. 17 ( limited preview in Google Book search).
- ↑ Regulation (EC) No. 2076/2002 of the Commission of November 20, 2002 (PDF) extending the deadline according to Article 8 (2) of Council Directive 91/414 / EEC and on the non-inclusion of certain active substances in Annex I of this Directive and the revocation of the approval of plant protection products with these active substances
- ↑ Directorate-General for Health and Food Safety of the European Commission: Entry on flucythrinate in the EU pesticide database ; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 24, 2016.

