Flupirt
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
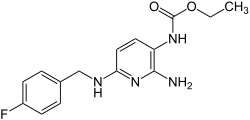
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Flupirt | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 15 H 17 FN 4 O 2 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| Drug class | |||||||||||||
| Mechanism of action |
Inhibits the transmission of pain by stabilizing the resting membrane potential |
||||||||||||
| properties | |||||||||||||
| Molar mass | 304.32 g mol −1 | ||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Flupirtine is a drug from the group of centrally acting, non-opioid analgesics. It has both a pain reliever ( analgesic ) and muscle relaxant effect .
Flupirtine has been approved for the treatment of acute pain in adults when treatment with other analgesics is contraindicated. After the Pharmacovigilance Committee (PRAC) of the European Medicines Agency (EMA) recommended in February 2018 that the approval of drugs containing flupirtine should be revoked due to the hepatotoxic potential, the suppliers withdrew their products from the market in the relevant EU countries.
pharmacology
As a centrally effective analgesic, flupirtine attacks the postsynaptic membrane . It does not work by influencing the glutamate release / transmission like opioids , but instead selectively opens inward potassium channels (“GIRK”, G-protein regulated inwardly rectifying K + channels ) on the postsynaptic membrane. This stabilizes the resting membrane potential so that the transmission of pain is inhibited. A greater pain stimulus and thus an increased glutamate release is required in order to trigger an action potential on the postsynaptic membrane and thus to transmit the pain.
The muscle relaxing effect is based on the same principle. However, it is not certain whether another, previously unknown mechanism for analgesia does not exist.
The analgesic potency of flupirtine is roughly between that of codeine and morphine .
Side effects
The most common symptoms are tiredness and dizziness, and gastrointestinal symptoms such as heartburn , nausea or diarrhea can occur. Drug-induced hepatitis up to liver failure occurs very rarely. Allergic reactions such as skin rash or itchy skin ( pruritus ) are possible. In individual cases, the use of flupirtine can lead to the development of addiction.
The Medicines Commission of the German Medical Association (AKdÄ) reported in March 2013 on liver damage from flupirtine. The European Medicines Agency (EMA) initiated a risk assessment process for flupirtine. The procedure was based on an application from the Federal Institute for Drugs and Medical Devices (BfArM), which had around 950 reports on adverse drug reactions. In addition, the BfArM saw the effectiveness of flupirtine in chronic pain as insufficiently proven. As a result, in July 2013, after the liver toxicity risk was assessed, the therapeutic use was restricted in terms of target group and duration of treatment, and further measures were ordered to reduce the risk of serious liver damage.
In February 2018, the EMA recommended that the marketing authorizations be withdrawn, as the risk for the patients predominates and therapeutic alternatives are available. The revocation took place in Germany in April 2018. In addition to Germany, the countries of the Baltic States, Luxembourg, Poland, Portugal and Slovakia are affected by the EMA recommendation.
Interactions and restrictions on use
Flupirtine binds strongly to serum proteins and is able to displace other protein-bound drugs from their binding, in particular warfarin and diazepam , the effect of which is thereby increased. The effects of alcohol , muscle relaxants and other sedative drugs can also be increased.
Flupirtine is extensively metabolised by the liver, which must be taken into account when substances with known and clinically important hepatotoxicity (e.g. carbamazepine , paracetamol , alcohol) are used at the same time .
history
Flupirtin was developed by the chemist Walter von Bebenburg for the company Asta Medica AG (Frankfurt a. M.). Bebenburg became better known as a writer under his pseudonym Walter E. Richartz .
Trade names
Flupirtine was available as a single preparation in capsules containing 100 mg of flupirtine maleate each or as a sustained- release formulation with 400 mg of flupirtine maleate.
Katadolon S long (D), Katadolon (D), Trancolong (D), Trancopal Dolo (D), Flupigil (D), Antidol (A), various generics (D) - all except trade (aH).
Individual evidence
- ↑ a b J. Kornhuber, S. Bleich, J. Wiltfang, M. Maler, CG Parsons: Flupirtine shows functional NMDA receptor antagonism by enhancing Mg2 + block via activation of voltage independent potassium channels. In: J. Neural Transm. Volume 106, No. 9-10, 1999, pp. 857-867, PMID 10599868 , doi: 10.1007 / s007020050206
- ↑ a b c T. Herdegen: Short textbook pharmacology and toxicology. Thieme, Stuttgart / New York 2008, page 287.
- ↑ a b data sheet Flupirtine maleate salt from Sigma-Aldrich , accessed on July 17, 2017 ( PDF ).
- ↑ a b Drugs containing flupirtine: Implementation of the implementation decision of the EU Commission. BfArM , January 27, 2014, archived from the original on December 22, 2017 ; Retrieved July 17, 2017 .
- ↑ a b PRAC recommends that the marketing authorization of the painkiller flupirtine be withdrawn. (PDF; 69 kB) European Medicines Agency , February 9, 2018, accessed on February 15, 2018 .
- ^ Mathias Schneider: Manufacturers are taking flupirtine-containing drugs off the market . In: DAZ.online . February 21, 2018 ( deutsche-apotheker-zeitung.de [accessed March 15, 2018]).
- ↑ a b M. Strohmeier: Flupirtine: effect, areas of application, side effects. January 19, 2019, accessed March 22, 2020 .
- ↑ Technical information Katadolon 100 mg hard capsules , as of October 2008, AWD.pharma GmbH & Co. KG. Available on the portal of the federal and state governments, PharmNet.Bund
- ↑ C. Stoessel, A. Heberlein, T. Hillemacher, S. Bleich, J. Kornhuber: Positive reinforcing effects of flupirtine - two case reports . In: Progress in Neuro-Psychopharmacology & Biological Psychiatry . tape 34 , no. 6 , 2010, p. 1120-1121 , doi : 10.1016 / j.pnpbp.2010.03.031 , PMID 20362025 .
- ↑ Liver damage caused by flupirtine (PDF; 127 kB) From the ADR database of the Drugs Commission of the German Medical Association (AKdÄ), accessed on March 5, 2013.
- ↑ Flupirtin: European risk assessment process started. BfArM , March 15, 2013, archived from the original on December 22, 2017 ; Retrieved July 17, 2017 .
- ↑ Drugs Commission of the German Medical Association (AkdÄ): Rote-Hand-Brief Restriction of the therapeutic target group and limitation of the duration of treatment after assessment of the liver toxicity risk , (PDF; 369 kB).
- ↑ Drug Safety Mail 2018-08 - Information from the EMA on flupirtine-containing drugs: PRAC recommends withdrawal of the marketing authorization. AkdÄ , February 9, 2018, accessed February 10, 2018 .
- ↑ Flupirtin: Recall and revocation of the approval of the pain reliever Flupirtin , AKDAE report from June 5, 2018, accessed on June 22, 2018.
- ↑ Finally: Off for Flupirtin in sight ... at demands immediate, independent market withdrawal from the providers , arznei-telegram, February 16, 2018.
- ↑ Heribert Offermanns : WE Richartz, W. von Bebenburg - writer and chemist . In: Chemistry in Our Time . tape 46 , no. 3 , 2012, p. 158–159 , doi : 10.1002 / ciuz.201200591 .