Gallionella
| Gallionella | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
Gallionella ferruginea |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name of the family | ||||||||||||
| Gallionellaceae | ||||||||||||
| Henrici & Johnson 1935 | ||||||||||||
| Scientific name of the genus | ||||||||||||
| Gallionella | ||||||||||||
| Ehrenberg , 1838 |
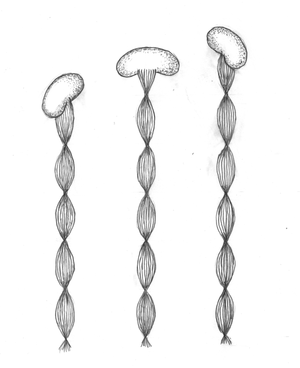
Gallionella is a genus of bacteria that cannot beclassifiedreliably in the phylogenetic system of bacteria and is currently listed as the only genus of the Gallionellaceae family in the order Nitrosomonadales. With Gallionella ferruginea a representative of this genus was described for the first time in 1836. Gallionella is bean-shaped, gram-negative , polar monotrich flagellated, gains energy from the oxidation of divalent iron ions with oxygen ( chemolithotrophy ), is carbon autotrophic and neutrophilic (occurs in the middle pH range). Characteristic of these organisms are the twisted appendages that are formed in the middle of the body on the concave side. However, these cell attachments are not found in all gallionella .
Energy metabolism
Gallionella uses as a power source which in the oxidation of divalent iron - ions (Fe 2+ with) oxygen (O 2 to give compounds of trivalent iron energy released) (Fe (III)). Sum equation for the oxidation of Fe 2+ ions to Fe 3+ ions:
- Fe 2+ + 1/4 O 2 + H + → Fe 3+ + ½ H 2 O
- Fe 3+ + 3 H 2 O → Fe (OH) 3 + 3 H +
The sum equation for the oxidation of Fe 2+ to Fe (OH) 3 is:
- Fe 2+ + 1/4 O 2 + 2½ H 2 O → Fe (OH) 3 + 2 H +
The prerequisite for the possibility of obtaining energy from the Fe 2+ oxidation is that the chemical equilibrium of the redox reaction is on the side of the trivalent iron in the medium , because otherwise no energy would be released during the conversion, but on the other hand the Fe 2+ ions must be largely stable so that they are available to the bacterium. The activation energy of the Fe 2+ oxidation must therefore be so high that a running self-oxidation ( autoxidation ) runs very slowly. In the case of high oxygen concentrations, high redox potential and high pH value, this is not the case; under these conditions auto-oxidation takes place quickly and there is no possibility for the bacterium to gain energy through oxidation. At a relatively low oxygen concentration and a pH value that is not too high, however, Fe 2+ is stable because the activation energy of the oxidation to Fe (III) is high. Under these conditions, the bacterium can enzymatically catalyze the oxidation , ie reduce the activation energy and direct it so that the energy released can be used to meet its energy requirements. The change in the free energy under standard conditions ΔG 0 ′ in iron oxidation is hardly suitable for estimating the energy released, because the concentrations of the reactants differ greatly from the standard conditions under natural conditions as well as under laboratory conditions, especially the concentration of iron (III) Product, which is extremely low due to its very low water solubility. This fact is favorable for the energy gain. For real conditions, a ΔG of -62.5 kJ / mol was calculated.
particularities
In the middle pH range , in which Gallionella occurs, the Fe 3+ ions are not stable, but they react with water to form almost insoluble Fe (III) compounds, which appear as brown precipitates, for example to iron ( III) hydroxide : The excretion of the oxidation product in the form of a band in a narrowly defined area of the cell surface has the advantage that the bacterial cell is not surrounded by the iron precipitates and that in this way the exchange of substances with the environment is not hindered. The band consists of about 40 (or more) parallel filaments , which in turn consist of globular particles strung together with a diameter of about 40-300 nm, and is twisted around its longitudinal axis. The twist may be caused by the ligament's outer fibrils being slightly longer than the inner ones. It is assumed that the primarily excreted band consists of ferrihydrite (a ferric oxide hydrate of unclear structure). Most of the time, the tape is attached to a solid body and the bacterial cell is therefore moved further while rotating by extending the tape. When the bacterium divides, the ligament is divided and each of the two daughter cells continues a branch of the ligament so that the ligament is dichotomously branched at the point of division . If the band ages, it is thickened by the abiotic deposition of further iron (III) oxide hydrates.
Occurrence
The occurrence is determined by the need to obtain energy from the oxidation of iron. This means that the environment must contain Fe 2+ ions and oxygen. This is the case in habitats in which water inflows from anoxic , iron-containing areas, e.g. from the groundwater area containing iron-containing subsoil. In such areas, because of the low redox potential, iron is present in the form of Fe 2+ ions dissolved in the water . The required oxygen enters the habitat through contact with air. The oxygen concentration, the redox potential and the pH value must, however, be in ranges in which the equilibrium of the redox reaction is on the Fe (III) side, but the activation energy is so high that the autoxidation proceeds very slowly (see under Energy Metabolism ). That is why Gallionella occurs at redox potentials from +200 to +320 mV, at oxygen concentrations from 0.1 to 1 mg / L and in the pH range from 6.0 to 7.6. In addition, CO 2 must be present (concentration above about 20 mg / L, because that is required by Gallionella for C-autotrophic growth. In Gallionella habitats, the Fe 2+ concentration is between about 5 and 25 mg / L, the concentration at organic matter is very low. The temperature range is mostly around 8 - 16 ° C, but there are also occurrences in warmer habitats, for example at thermal springs up to 47 ° C. Gallionella mostly occurs in non-haline waters ( fresh water ), but also in salt water habitats (sea water ) in front.
Growth, multiplication, distribution
The Gallionella cells grow in length and divide across. In a natural location, the generation duration (time between two cell divisions) was determined to be around 17 hours. In stationary liquid cultures, a generation time of about 11 hours was achieved. In both cases, just under 0.5 mm of tape was produced between two cell divisions. The distribution is evidently monopolar, monotrich flagellated "swarm cells" formed.
Technical importance
In wells for water extraction and in drainage systems in agricultural soils - if iron-containing groundwater is present - blockages are often caused by the mass development of Gallionella and the associated precipitation of Fe (III) compounds.
When iron-containing groundwater is treated to make drinking water, the dissolved iron must be removed from it. This is done by introducing oxygen from the air and separating the iron as Fe (III) compounds in filters. A population of Gallionella develops without which iron precipitation would only take place slowly and would lead to insufficient iron removal from the groundwater.
species
So far only one species is known for certain, namely Gallionella ferruginea . However, other species have also been described, mainly due to variations in the shape of the ribbon. For example, a species of G. filamenta has been described in which the band consists of only 3 - 8 parallel filaments. However, the identity of these forms as independent species is not certain.
literature
- George M. Garrity, Julia A. Bell, Timothy G. Lilburn: Taxonomic Outline of the Prokaryotes. Bergey's Manual of Systematic Bacteriology . Second Edition, Release 5.0, Springer-Verlag, New York, 2004. PDF .
- Martin Dworkin, Stanley Falkow, Eugene Rosenberg, Karl-Heinz Schleifer, Erko Stackebrandt (Eds.) The Prokaryotes, A Handbook of the Biology of Bacteria . Volume 5: Proteobacteria: Alpha and Beta Subclasses , 3rd edition, Springer-Verlag, New York et al. O., 2006, ISBN 978-0-387-25495-1 (print) and ISBN 978-0-387-30743-5 (Online), doi : 10.1007 / 0-387-30743-5 .
Web links
swell
- ↑ JP Euzéby: List of Prokaryotic names with Standing in Nomenclature - Family Gallionellaceae
- ↑ CG Ehrenberg: Preliminary reports on the real occurrence of fossil infusoria and their wide distribution . In: Poggendorf's annals of physics and chemistry . Vol. 38, 1836, pp. 213-227.
- ^ Arnold Martin Gebauer: Detection and quantification of the autotrophy metabolism of Gallionella ferruginea Ehrenberg . PhD dissertation at the Technical University of Braunschweig 1993.
- ↑ FV Tschukrov, LP Ermilova, AI Gorschkov, BB Zviagin, AP Shukchlistov, OW Siderenko, VV Balashova: About the nature of iron oxides in geologically young formations . In: Chemie der Erde Vol. 33, 1974, pp. 109-124.
- ↑ Helmut Hanert: Structure and growth of Gallionella ferruginea Ehrenberg in the natural location in the first 6 hours of development . In: Archives for Microbiology . Vol. 75, 1970 pp. 10-24.


