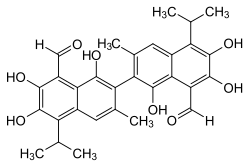
Gossypol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Gossypol | ||||||||||||||||||
| other names |
2,2′-bis (formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene) |
||||||||||||||||||
| Molecular formula | C 30 H 30 O 8 | ||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 518.56 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Gossypol is a yellow-reddish dye . It is an atropisomeric aromatic dimeric sesquiterpene that is slightly toxic to humans because it inhibits the enzyme lactate dehydrogenase . Gossypol consists of two mirror image isomeric forms ( enantiomers ) and has axial chirality .
Occurrence
Gossypol is about 1.5 percent in the seeds of the cotton plant .
Medical research
Gossypol is a research object in cancer research. Initial laboratory tests showed that Gossypol blocks certain proteins in cancer cells and thus their cell division. One of the variants in particular showed good test results.
In China, research was carried out with Gossypol until the 1970s in order to obtain a contraceptive for men. Since 20% of the test persons remained permanently sterile, the WHO recommended in 1998 that research on the “ pill for men ” with Gossypol be stopped.
Individual evidence
- ↑ a b entry on Gossypol. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ↑ a b c data sheet Gossypol from cotton seeds from Sigma-Aldrich , accessed on April 3, 2011 ( PDF ).
- ^ Römpp Lexicon Natural Products, page 270.
- ^ ST Page, JK Amory, WJ Bremner: Advances in male contraception. In: Endocrine reviews. Volume 29, Number 4, June 2008, pp. 465-493, ISSN 0163-769X . doi : 10.1210 / er.2007-0041 . PMID 18436704 . PMC 2528850 (free full text). (Review).
- ↑ GM Waites, C. Wang, PD Griffin: Gossypol: reasons for its failure to be accepted as a safe, reversible male antifertility drug. In: International journal of andrology. Volume 21, Number 1, February 1998, pp. 8-12, ISSN 0105-6263 . PMID 9639146 . (Review).
Web links
Edible Cotton - Technology Review - Article on cotton with genetically reduced gossypol content

