Instrument-based analytics
The instrumental analysis (IA) is the field of analytical chemistry which carries out the analysis and identification of unknown substances ( "sample") or their molecular structures by means of modern analyzers. For this purpose, a sample is usually prepared (e.g. by extracting and eluting ), placed in the device and irradiated or disassembled in order to record certain measured values in a detector. These indicate the type, concentration or amount of the unknown substance - often down to tiny amounts.
Development and characteristics

The instrumental analysis is based on high-precision measuring principles, which have been newly developed over the last 40 years in order to be able to counter the explosively increased requirements from research, environmental analysis and product control. The handling of the devices (laboratory technology) was also further developed during this period. Compared to classic wet chemical methods, it is the characteristics of instrumental analysis methods that measurements can be carried out quickly, inexpensively, correctly, precisely, accurately and with the smallest quantities with the help of state-of-the-art analysis equipment. Many substances (test substances) have thus become measurable down to the range of a few picograms that is hardly imaginable for laypeople .
An exact description of the respective instrumental analysis method (for example, when testing a ready-made meal for a pesticide in vegetables) includes the test equipment (the laboratory ) and the test system (here: the ready-made meal), the details of the test device (e.g. gas chromatograph, GC), the test substance (the pesticide ) and the sample (brand of the ready-made meal "Gypsy schnitzel with mashed potatoes", production of the extract from e.g. 10 g paprika with the help of a certain extractant such as 50 ml dichloromethane ).
The quality of an analytical method is described by documenting sample preparation (e.g. by elution or extraction) and method validation, i.e. evidence and documentation of the reliability of an analytical method. For this purpose, the correctness, the accuracy and the precision of the method are examined and, in the case of the accuracy, also calculated with the aid of the Gaussian function (e.g. when displaying measured values in " peak " form). The calibration establishes a connection between the measured variable and the analysis result, the reliability of the result is described by the arithmetic mean ("average"), the standard deviation and the recovery rate.
Classification
Four groups of procedures fall under the generic term instrumental analysis: optical, spectroscopic, chromatographic and electroanalytical methods.
Optical analysis methods
→ Introductory Article: Optics
Optical methods and devices are those in which the sample is illuminated with light or light-like electromagnetic radiation. Some of this radiation is bent or reflected at a certain angle. This can then be measured in a refractometer (Fig. Left).
In addition to refractometry (measurement of the refractive index ), these methods also include polarimetry (measurement of the optical activity / rotation value) and photometry (measurement of light absorption in the form of extinction or transmission at a certain wavelength). The polarimetry z. B. is a method in which a sample solution is transilluminated with linearly polarized light of a certain wavelength. Certain substances have the ability to rotate the plane of oscillation of linearly polarized light (specific rotation, optical activity). This specific rotation is often used in pharmacy and chemistry for the identification and purity control of chiral substances. Specifying the specific rotation for natural substances , such as amino acids , terpenes and sugars , is particularly important , since the majority of these substances are optically active.
Spectroscopic methods
In spectroscopic methods and devices, the sample interacts with electromagnetic radiation ( absorption and emission ). The various instrumental analysis methods in spectroscopy are divided into:
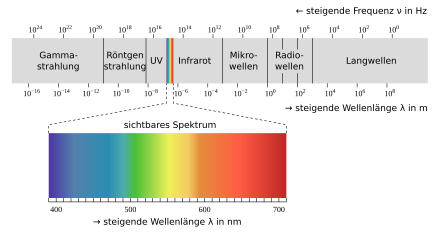
- the wavelength range of the radiation used (e.g. X-ray, UV, VIS and IR spectroscopy, see figure),
- the form of energy (e.g. nuclear magnetic resonance / NMR, electron spin or vibration spectroscopy),
- the examination technique (absorption or emission spectroscopy, e.g. AAS / AES).
The atomic spectroscopy in the atomic absorption spectrometer (abbreviation: AAS) is generally a measurement of the wave number, wavelength or frequency of absorbed radiation to determine atomic energy levels, the atomic emission spectroscopy in the atomic emission spectrometer / flame photometer (AES) measures radiated energy, the IR spectroscopy (in the infrared spectrometer IR) and the UV / VIS spectroscopy (in the UV / VIS spectrometer) measure in the infrared, visible and ultraviolet range of the electromagnetic spectrum.
A fluorescence spectroscopy measures the light emitted by a fluorescent sample in the fluorescence spectrometer, while an X-ray fluorescence analysis (XRF) uses X-rays. The nuclear magnetic resonance (NMR) is measured in the nuclear magnetic resonance spectrometer, the electron spin resonance in the electron spin resonance spectrometer (ESR or EPR).
The mass spectrometer (MS), on the other hand, is a device that does not work spectroscopically (no use of electromagnetic radiation), but breaks down the molecules of the sample substance into fragments, which are then captured by a magnetic field and sorted according to mass numbers.
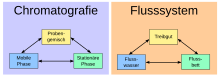
Chromatographic methods
Chromatographic methods and devices aim to set the sample (a mixture of substances) in motion under certain conditions so that the components can be divided up and identified based on different flow or migration speeds (gas, high-pressure liquid chromatography, etc.) similar, separation by means of adsorption and desorption and different retention times of the individual components). It is therefore a matter of high-precision micro-processes for material separation (compared to the classic macro-material separation processes of distillation, sublimation, extraction, recrystallization, reprecipitation and filtration).
Important analytical devices for chromatography are gas chromatographs (GC, often for determining peak areas and retention factors or times of certain substances), HPLC devices (for high-performance liquid chromatography ), ion chromatographs (for ion chromatography / electrophoresis IC / EP) and electrophoresis devices for gel electrophoresis , especially 2D -Gel electrophoresis and IEF gel electrophoresis (IEF = isoelectric focusing ).
Electroanalytical and other instrumental analysis methods
There are also electroanalytical and other physical methods and devices in which the sample z. B. electrically charged, exposed to the electric current ( electrolysis , potentiometry or conductometry, etc.) or broken up into molecular fragments (fragments) and separated (mass spectrometry MS). The nine most important procedures in this group are:
- Electrogravimetry to determine the concentration of the deposited electrolyte mass
- Potentiometry (voltammetry) for measuring the electrochemical potential or voltage
- Polarography / voltammetry recording of current-voltage curves for quantitative and qualitative analysis
- Amperometry Registration of the electrolysis current at constant potential
- Coulometry Registration of the electrical charge that is transferred during electrolysis
- Conductometry , e.g. B. as automated conductivity titration
- Osmometry to measure the osmotic pressure
- Viscometer for measuring viscosity
- Mass spectrometry to determine the molar mass of a molecule or its key fragments
Wet chemical or classical analysis methods such as volumetry (dimensional analysis) and gravimetry (precipitation analysis) are only counted among the instrumental analysis methods if electronic measuring instruments are used. In the case of dimensional analysis, this would be the instrumental indication of the equivalence point, for example by means of potentiometry or conductometry . An instrumental variant of gravimetry is thermogravimetry .
Coupled methods
The direct coupling of different analytical methods has recently gained in importance. A classic example is the so-called GC-MS , in which a mass spectrometer (MS) is connected to a gas chromatograph (GC) (GC-MS). The chromatograph achieves a separation of an often complex mixture of substances, while the mass spectrometer, continuously fed by the gas flow of the chromatograph, enables the individual sample components to be identified. Similar combinations are the coupling of high performance liquid chromatography (HPLC) and mass spectrometry or of HPLC and NMR.
Analysis technology industry
The world market for analysis technology and laboratory equipment amounted to around USD 45 billion in 2013.
The 330 German manufacturers and their almost 40,000 employees generated sales of € 6.7 billion in the same year. Domestic sales amounted to € 3.1 billion and foreign sales to € 3.6 billion. Accordingly, the export quota was 54%.
The most important customer sectors for German manufacturers are currently industry, the public sector and the pharmaceutical and chemical sectors. Around 85 percent of domestic sales are generated in these markets. But there are also numerous other sectors and niches in which the companies are successfully asserting themselves. Examples of this are the areas of biotechnology and food.
The most important target countries for German exports of analysis and laboratory technology include the USA, China, France, the United Kingdom, Italy, the Russian Federation, the Republic of Korea, Japan, the Netherlands and Switzerland.
literature
- Heinz Hug: Instrumental Analytics - Theory and Practice. Haan-Gruiten 2010, ISBN 978-3-8085-7211-5 .
- Michael Wächter: chemistry laboratory. Introduction to laboratory practice. 1st edition. Wiley-VCH, Weinheim 2011, ISBN 978-3-527-32996-0 , Chapter 5.3: Instrumental analysis
- Michael Wächter: Book of tables of chemistry. Data on analysis, laboratory practice and theory. 1st edition. Wiley-VCH, Weinheim 2012, ISBN 978-3-527-32960-1 , Chapter 9: Analytics
- Wolfgang Gottwald: Instrumental-analytical internship. Wiley-VCH, Weinheim / New York / Basel / Cambridge / Tokyo 1996, ISBN 3-527-28755-8 .
- Wolf R. Less, Stefan Eckhardt and others: The action-oriented training for laboratory occupations. Volume 2: Elective Qualifications. Würzburg 2006, ISBN 3-8343-3021-3 , pp. 87-378.
- Douglas A. Skoog, James J. Leary: Instrumental Analytics: Fundamentals - Devices - Applications. Berlin 1996, ISBN 3-540-60450-2 .
Individual evidence
- ↑ BfR Infographic. In: Federal Institute for Risk Assessment. Retrieved August 10, 2019 .
- ↑ Infographic - The Incredible Progress in Analytical Chemistry. In: German paint institute. Retrieved August 14, 2019 .
- ^ The German Industry for Analysis, Biotechnology and Laboratory Technology - Yearbook 2014. Spectaris