Isoproturon
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Isoproturon | |||||||||||||||
| other names |
3- (4-isopropylphenyl) -1,1-dimethylurea |
|||||||||||||||
| Molecular formula | C 12 H 18 N 2 O | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 206.29 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.16 g cm −3 |
|||||||||||||||
| Melting point |
156.5-158 ° C |
|||||||||||||||
| Vapor pressure |
0.000003106 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Isoproturon is a chemical compound from the group of urea derivatives .
Extraction and presentation
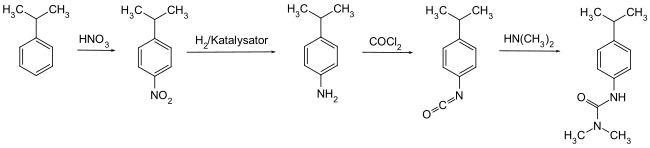
Isoproturon can be obtained from cumene by nitration in the para position, reduction to 4-isopropylaniline , reaction with phosgene to form 4-isopropylphenyl isocyanate and its subsequent reaction with dimethylamine .
properties
Isoproturon is a colorless and odorless flammable solid that is sparingly soluble in water. It decomposes when heated. It is very stable to light, acids and bases. When heated in strong bases, hydrolytic cleavage occurs .
use
Isoproturon is used as an active ingredient in crop protection products. It is preferred as a herbicide for winter wheat, winter barley, rye, spring barley and spring wheat against weed grasses ( field foxtail grass , common wind stalk , various bluegrass, as well as chamomile and chickweed ) (both as pre- and post-emergence herbicide). Isoproturon is used very little or not at all for spring barley and oats, potatoes, rapeseed, sugar beet or corn. The annual consumption in Germany is over 1000 tons.
Admission
Isoproturon was in the GDR between 1980 and 1994 and has been approved in the FRG since 1975. With effect from the beginning of 2003, isoproturon was approved for use as a herbicide in the European Union . With the exception of Denmark and Finland, preparations containing isoproturon were approved in all EU member states and Switzerland (e.g. under the trade names Arelon and Azur).
After the European Commission decided not to extend the approval for isoproturon as a plant protection product, which ended on June 30, 2016, the approval in Germany was revoked on September 30, 2016. After the revocation, a sell-off period for stocks was valid until March 30, 2017 and a use-by period until September 30, 2017. It was regularly detected in German and Dutch rivers from 2000 to 2002. In Germany, Austria and Switzerland, plant protection products (e.g. Arelon, Azur) with this active ingredient were approved. Preparations containing isoproturon were approved in all EU member states, except Denmark and Finland. The approval of plant protection products with this active ingredient was revoked in Germany on September 30, 2016. After the revocation, there is a sell-off period for stocks until March 30, 2017 and a use-by period until September 30, 2017. It was regularly detected in German and Dutch rivers in the years 2000 to 2002.
Individual evidence
- ↑ a b c d e f g h i j Entry on isoproturon in the GESTIS substance database of the IFA , accessed on November 18, 2019(JavaScript required) .
- ↑ a b Isoproturon data sheet , PESTANAL at Sigma-Aldrich , accessed on May 18, 2017 ( PDF ).
- ^ S. Gangolli (Ed.): The Dictionary of Substances and Their Effects. Volume 4 EJ 2nd edition, Royal Society of Chemistry, 1999, p. 877.
- ↑ Robert Krieger: Handbook of Pesticide Toxicology . Academic Press, 2001, ISBN 0-12-426260-0 , pp. 1525 (English).
- ↑ Entry on Isoproturon (ISO); 3- (4-Isopropylphenyl) -1,1-dimethylurea in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on November 18, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 239 (English, limited preview in Google Book search).
- ↑ a b Emission reduction for priority and priority hazardous substances of the Water Framework Directive - material data sheets - data sheet Isoproturon , texts 29/07, environmental research plan of the Federal Minister for the Environment, Nature Conservation and Nuclear Safety, p. 242ff.
- ↑ Peter Brandt: Reports on Plant Protection Products 2009: Active Ingredients in Plant Protection Products , Approval History and Regulations of the Plant Protection Application Ordinance . Springer DE, 2010, ISBN 3-0348-0029-0 , p. 19 ( limited preview in Google Book search).
- ↑ Directive 2002/18 / EC of the Commission of February 22, 2002 amending Annex I of Directive 91/414 / EEC of the Council on the placing on the market of plant protection products containing the active substance isoproturon .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on isoproturon in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on July 16, 2019.
- ↑ Gerrit Hogrefe: Important building blocks in the herbicide range are missing . In: agrarzeitung . July 15, 2016, p. 18 .
- ↑ a b bvl.bund.de: BVL - Fachmteilungen - Approvals of pesticides with isoproturon and triasulfuron will be revoked on September 30, 2016 , accessed on July 23, 2016
- ↑ IKSR CIPR ICBR ICPR: 135th synthesis report on isoproturon and chlorotoluron. Retrieved June 13, 2019 .
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on isoproturon in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on July 16, 2019.
- ↑ Gerrit Hogrefe: Important building blocks in the herbicide range are missing . In: agrarzeitung . July 15, 2016, p. 18 .
- ↑ IKSR CIPR ICBR ICPR: 135th synthesis report on isoproturon and chlorotoluron. Retrieved June 13, 2019 .