JWH-018
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | JWH-018 | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 24 H 23 NO | |||||||||||||||
| Brief description |
white crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action |
Cannabinoid receptor CB 1 / CB 2 agonist |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 341.45 g mol −1 | |||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
soluble in acetone |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
JWH-018 is a synthetic chemical compound from the group of alkyl indol - derivatives . It is a cannabinoid receptor - CB 1 / CB 2 - agonist with cannabinoid mimetic effect.
In animal experiments, an effect similar to tetrahydrocannabinol (THC) was found, the duration of the effect being shorter. According to its developer John W. Huffman (JWH is derived from his initials), there have not yet been any long-term studies or investigations into how this substance affects other living things than mice.
The synthetic molecule developed at Clemson University in South Carolina is an attempt to find a commercially interesting alternative to the medicinal use of THC . THC is used, among other things, as an appetite stimulant for certain diseases, and it can also reduce side effects of chemotherapy such as vomiting in cancer patients.
Occurrence
On December 15, 2008, a study was published in which JWH-018 was found as part of the herbal mixture Spice sold as an incense .
Extraction and presentation
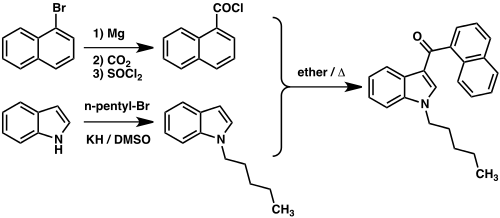
JWH-018 can be prepared by Friedel-Crafts acylation of 1-pentylindole and naphthalene-1-carbonyl chloride. Naphthalene-1-carbonyl chloride is obtained by reacting 1-naphthalenecarboxylic acid (obtainable from 1-bromonaphthalene by means of a Grignard reaction with CO 2 ) with thionyl chloride. 1-Pentylindole is made from indole and 1-bromopentane .
Legal position
- Germany:
- As an active ingredient , JWH-018 was classified in Germany as a marketable but not a prescription narcotic by entry in Annex II of the Narcotics Act (BtMG) as of January 22, 2009 . Limited to one year, any form of unauthorized manufacture, trade and possession was prohibited. On January 14, 2015, the German Federal Supreme Court ruled on the not insignificant amount of various synthetic cannabinoids . The limit of the not small amount was set at two grams for the cannabinoids JWH-018 and CP 47,497-C8 homologs.
- Austria
- JWH-018 is not subject to the Austrian narcotics law. However, the sale of "smoking mixtures" with synthetic cannabinoids has been banned since December 18, 2008 in accordance with Section 1, Paragraph 1, Item 5 of the Medicines Act . Furthermore, since February 1, 2012, the substance has been subject to the "Federal Act on Protection against Health Risks in Connection with New Psychoactive Substances" (New Psychoactive Substances Act, NPSG), provided it is intended for human consumption.
- Switzerland:
- With the entry into force of the revised Narcotics Ordinance by Swissmedic on December 1, 2010, JWH-018 became subject to the Narcotics Act and has therefore been illegal since then. Importation, possession, distribution etc. are punished according to the Narcotics Act.
See also
Individual evidence
- ↑ a b Justin Denton: One-Pot Desulfonylative Alkylation of N-Sulfonyl Azacycles Using Alkoxides Generated by Phase-Transfer Catalysis. In: Synthesis . 2010, pp. 775-782, doi : 10.1055 / s-0029-1218627 .
- ↑ SWGDRUG Monographs: JWH-018 (PDF; 363 kB), accessed on May 20, 2013.
- ↑ a b WHO: JWH-018. Critical Review Report p. 9
- ↑ There is not yet a harmonized classification for this substance . A labeling of 3- (naphthalene-1-carbonyl) -1-pentyl-1H-indole in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on November 11, 2019, is reproduced from a self-classification by distributors .
- ↑ John W. Huffman et al. (2005): 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. In: Bioorg. Med. Chem. Lett. 15 (18): 4110-4113. PMID 16005223 doi : 10.1016 / j.bmcl.2005.06.008 .
- ↑ Blog entry by Pierre Markuse: Spice: JWH-018 - Email from Prof. John W. Huffman .
- ^ Christian Steup: Investigation of the commercial product "Spice". THC PHARM GmbH. PDF
- ^ Frankfurter Rundschau Online: Dangerous kick with "Spice". ( Memento of March 16, 2010 in the Internet Archive ) December 15, 2008.
- ↑ BGBl I No. 3 of January 21, 2009, 22nd BtMÄndV of January 19, 2009, pp. 49-50.
- ↑ BGH, judgment of January 14, 2015 - 1 StR 302/13.
- ↑ Juris.de: Legal Highs - Limits for synthetic cannabinoids set , accessed on January 24, 2015.
- ↑ Ordinance of the Swiss Agency for Therapeutic Products on Narcotic Drugs and Psychotropic Substances (Narcotics Ordinance Swissmedic, BetmV-Swissmedic.) Amendment of 10 September 2010 (PDF; 590 kB) Comes into force on 1 December 2010.
- ↑ Federal Act on Narcotics and Psychotropic Substances (Narcotics Act, BetmG) of October 3, 1951 (as of January 1, 2010) (PDF; 183 kB). Swiss Narcotics Act, relevant criminal provisions: Art. 19 and following.