Potassium hydrogen phthalate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
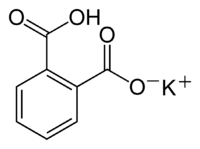
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Potassium hydrogen phthalate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 5 KO 4 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 204.22 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.64 g cm −3 |
||||||||||||||||||
| Melting point |
295–300 ° C (decomposition) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Potassium hydrogen phthalate (also called potassium acid phthalate) is a salt of o -phthalic acid . The colorless, crystalline solid is soluble in water.
properties
In water dissociates potassium hydrogen phthalate completely to form a potassium - cation (K + ) and a Hydrogenphthalat- anion (H P - ). As a weak acid, hydrogen phthalate reacts reversibly to a small extent with water to form oxonium (H 3 O + ) and a doubly negatively charged phthalate ion (P 2− ).
use
Potassium hydrogen phthalate is used as a standard reference material for pH measurement (pH 4.01) and as a buffer substance (in combination with hydrochloric acid or sodium hydroxide ). It is also used as a concentration standard for measurements of organically bound carbon (TOC) and related sum parameters of the organic pollution of water.
See also
Individual evidence
- ↑ a b c data sheet potassium hydrogen phthalate (PDF) from Carl Roth , accessed on August 24, 2017.
- ↑ a b c data sheet Potassium hydrogen phthalate from Sigma-Aldrich , accessed on August 24, 2017 ( PDF ).
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 89th edition. (Internet version: 2009), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-438.
- ↑ Data sheet pH buffer solution pH 4.01 (PDF) from Carl Roth , accessed on December 14, 2010.
