Corrosion protection
As corrosion protection measures are called for avoiding damage caused by corrosion can be caused by metallic components. Since absolute corrosion resistance cannot be achieved, the protective measures taken generally aim to reduce the speed of the corrosive attack to such an extent that damage to the component can be avoided during its service life.
General
The term corrosion is no longer only used for metallic materials, but also for glass, plastics, building materials, etc. The attacking medium is called a corrosive agent. If the functionality of a component is impaired as a result of a corrosion attack, this is referred to as corrosion damage.
According to DIN EN ISO 8044, a distinction is made between the following types of corrosion:
- Surface corrosion , in which the surface is damaged evenly
- Well corrosion , in which the surface is damaged unevenly and severely
- Pitting corrosion that only destroys small areas of the surface
- Crevice corrosion , in which the material surface in narrow gaps, z. B. in welds is attacked

In order to prevent the chemical corrosion processes, there are various methods and procedures that are generally referred to as corrosion protection. Metallic corrosion protection is discussed in more detail below.
A distinction is made between active and passive corrosion protection. Based on the term “constructive wood protection ”, a certain level of corrosion protection can also be achieved with metallic materials through a suitable construction.
Passive corrosion protection
Passive corrosion protection comprises all measures that achieve a shielding effect against corrosive media.
- The contact with corrosive media such as water can be reduced by means of constructive measures such as roofing, splash protection and a slope for draining liquids.
- A material can be almost completely isolated from corrosive gases and liquids by means of suitable coatings such as coatings . Binders such as synthetic resins are often with additives such as micaceous iron oxide - pigments enriched to improve the shielding effect and increase the resistance. Pigments such as zinc dust , zinc phosphate , zinc chromate or red lead are also referred to as active pigments because they also have a chemical or galvanic rust protection effect.
Synthetic resins such as EP or PU , plastics such as PVC and plastic films , oils , lacquer , rubber or hard paraffins are used as coating materials applied in liquid, paste or powder form .
Examples of (inorganic or metallic) coatings are a conversion layer by a phosphating , an anodized layer , a hard anodic oxide film , a chromate or other conversion layers with rather non-metallic character and hot-dip galvanizing , which in turn form a protective passivation layer and, moreover, in case of damage to the substrate as a sacrificial anode to protect .
Large and long pipelines made of steel for water transport are preferably lined with an inner coating of cement mortar to protect against corrosion . The corrosion resistance can be further improved by adding suitable plastics. The main advantages of this type of coating are:
- low cost
- largely resistant to corrosive water up to a pH value of about> 4.0; this resistance does not apply to deionized water .
- Self-healing of narrow cracks that go to the iron surface
Also, by electroplating , or chemically generated, metallic cover layers of tin , gold , nickel , copper , chromium , zinc or alloy coatings such as nickel-phosphorus ( chemical nickel ) or zinc-nickel bring about a corrosion protection. Also finds galvanizing (a hot-dip process) is widely used. The protective effect of metal layers is based on their property of not corroding themselves (precious metals) or on the barrier effect through the formation of a dense oxide layer on the surface (so-called passive layer), which serves as corrosion protection. Some metals are able to form a surface layer "by themselves" that protects the base material, such as the patina on copper or zinc.
With metallic layers, the protective effect in the event of layer damage depends on the type of layer. Less noble layers protect the workpiece cathodically and act as a sacrificial anode - the layer preferentially dissolves and thus maintains the function of the component for as long as possible. Even smaller defects or damage in the layer therefore initially have no serious effects (so-called remote effect). A classic example is the galvanizing of steel , but also the protection of hydraulic structures such as As ships, locks, sheet piling, boat parts and rails by attaching sacrificial anodes of zinc - aluminum - or magnesium - alloys . More noble layers than the base material protect it anodically, but have the major disadvantage that if the layer is damaged, the less noble base material underneath is dissolved more quickly ( contact corrosion ).
A certain amount of corrosion protection is offered by B. tinplate - here tin is used as a coating material so that food can also be packed with it. However, after some time, if the can is open, tin ions are formed, which are toxic to z. B. Cress work. Therefore, the can should also be painted .
In rust converters , corrosion protection is achieved by removing the original, porous iron oxides and further oxidizing the upper iron layer to form an Fe oxide with a smooth surface that prevents water from being absorbed and thus preventing further rusting. Phosphoric acid or tannin is used for this, mixed with additives. After the treatment, sealing with polymer varnishes is common in order to achieve permanent protection. Products are also offered that are intended to combine both functions.
Active cathodic corrosion protection
Active cathodic corrosion protection with external current
Another possibility for cathodic corrosion protection - KKS for short - can be achieved by means of external current and external current anodes . In the case of oil pipelines , z. B. at intervals of a few kilometers at a distance of a few hundred meters across the line electrodes sunk into the ground, which are fed with a counter voltage. The other pole is on the pipeline, so that this voltage gradient precisely compensates for the galvanic element made up of the ground and metal line, which is in the range of a few volts . Since this depends on the chemical composition of the soil, it must be examined and the counter-voltage fed in must be adapted to the local conditions.
In bridge construction , especially on motorway bridges, the KKS is carried out using an impressed current anode. For this purpose, an anode grid made of coated titanium is applied to the surface to be protected and about 2 cm to 3 cm is injected with shotcrete . The shotcrete serves as an electrolyte . The current is fed into the reinforcement via a rectifier, thus providing cathodic protection. The measure is continuously checked with an automatic monitoring system.
In addition, electrodes for cathodic protection from titanummanteltem copper ( English titanium clad copper ) as well as silver-silver chloride used.
Iron is more positive than zinc in the electrochemical series , ie zinc is less noble than iron and represents the anode in the galvanic element, and iron is the cathode. Iron, as a noble metal, is cathodically protected until the zinc has corroded away.
- Protection of a pipeline system with corrosion inhibitors that are added to the water cycle
- Standardized environmental tests are carried out to determine the corrosion resistance of coatings. Here, for example, surfaces are easily damaged with scratch test devices and then exposed to a salt spray .
Active cathodic corrosion protection without external current


The aim of active corrosion protection without external current is to protect a metal from rust that often comes into contact with water, for example. To do this, one uses a less noble substance that is sacrificed for the nobler metal. For corrosion protection using a sacrificial anode , anodes made of different materials are used depending on the application. The sacrificial anode must be conductively connected to the metal to be protected in order to achieve protection. A current of a few milliamps flows in the resulting circuit, which is fed from the redox reaction of the oxidation of this sacrificial anode .
In the case of iron and magnesium, the reaction proceeds as follows: As soon as magnesium or iron comes into contact with the water, it is oxidized to Mg 2+ or Fe 2+ . According to the electrochemical series, there is a potential difference between magnesium and iron of 1.9 V (standard potentials at 25 ° C; 101.3 kPa; pH = 0; ion activities = 1). Since magnesium, with a potential difference to hydrogen of –2.362 V, has a significantly more negative potential than iron with –0.41 V, magnesium is oxidized at the anode and the iron is reduced by accepting electrons. This reaction takes place very slowly, but can be accelerated by changing conditions. The incident on the water electron now divide this into H 2 and 2OH - on. The iron does not change because it can take up the electrons given off by the magnesium. The magnesium, on the other hand, gradually dissolves and must be renewed after it has been completely broken down.
The magnesium anode is positioned in the middle of spherical containers so that the potential assumes the same value on all surfaces of the container. In the case of cylindrical containers, the anode is attached in such a way that there is approximately the same distance from the container bottom as from the circular container walls. The anode is therefore shorter than the depth of the container. In many cases, the anode is installed insulated from the wall of the container, since otherwise a higher protective current would flow at the installation point, while the rest of the container would be less well protected. The circuit is then closed via a cable which is connected to the anode and the container wall on the outside of the container. An ammeter for direct current can also be looped into the cable, which measures the flowing current in the milliampere range and thus shows the functionality of the anode.
In order to protect ships made of steel from corrosion by the sea water, magnesium anodes are attached to the outside of the hull at regular intervals.
example
A simple attempt at corrosion protection:
- An unprotected iron nail is placed in acidified salt water. After a while, iron goes into solution and corrodes (if there is no sacrificial anode). Furthermore, hydrogen (H 2 ) is formed on the iron nail.
- The iron nail is protected with the less noble magnesium. A local element is formed in that magnesium (Mg) acts as an anode and thus sacrifices for iron (Fe). Here too, hydrogen H 2 is formed on the Fe cathode. The reason for this is the electron flow (e - ) from Mg to Fe, since Mg is less noble than iron and therefore has a greater reducing power.
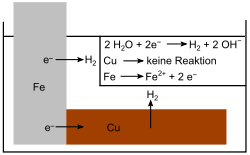
- If the nobler copper (Cu) is used instead of the magnesium, the iron acts as an anode and the copper cannot protect the iron from corrosion. The iron is oxidized faster than when no copper is present, because the electron flow now runs from Fe to Cu.
See also
- Hot-dip galvanizing
- Film galvanizing
- Society for Corrosion Protection
- Zinc flake coating
- Hot screw compound
- Sherardizing (diffusion galvanizing)
literature
- Bernhard Wietek : KKS in bridge repair . KKS seminar in Innsbruck 2000.
- Ulrich Bette, W. Vesper: Pocket book for cathodic corrosion protection . 7th edition. Vulkan, 2005, ISBN 3-8027-2932-3 .
- RP Gieler, A. Dimmig-Osburg: Plastics for building protection and concrete repair . Birkhäuser Verlag, Berlin 2006, ISBN 3-7643-6345-2 .
Individual evidence
- ↑ DIN EN ISO 12 944 part 1
- ^ B. Heinrich, H. Hildebrand, M. Schulze, W. Schenk, in: 3R international. 17th year, issue 7, July 1978, p. 455.
- ^ W. Schwenk, in: Zentralblatt für Industriebau. Volume 26, No. 5, September 1980, p. 309.
- ^ W. Schwenk, in: Zentralblatt für Industriebau. Volume 26, No. 5, September 1980, p. 308.
- ↑ hammerite.de: Technical data sheet for rust protection paint , accessed April 15, 2016.
- ↑ motipdupli.com: Technical data sheet Bob Rostversiegelung , accessed April 15, 2016.