L- lactate dehydrogenase
| L- lactate dehydrogenase | ||
|---|---|---|
|
||
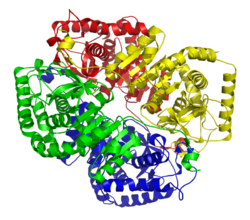
| Ligament model LDH-5 (muscle) according to PDB 1I10 | ||
| Mass / length primary structure | 333/331/331 amino acids | |
| Identifier | ||
| Gene name (s) | LDHA , LDHB , LDHC | |
| External IDs |
|
|
| Enzyme classification | ||
| EC, category | 1.1.1.27 , oxidoreductase | |
| Substrate | Pyruvate + NADH + H + | |
| Products | Lactate + NAD + | |
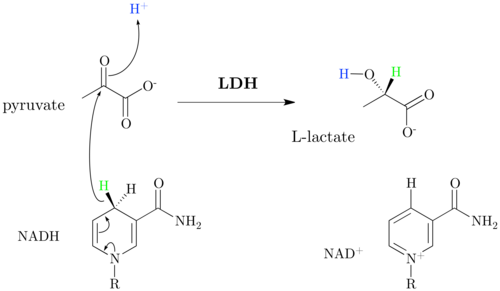
L -Lactatdehydrogenase or shortly lactate dehydrogenase ( LDH ) is an enzyme , the formation of L lactate and NAD + from pyruvate and NADH catalyzed. The reaction is reversible . LDH occurs in all cells of almost all living things . It is part of lactic acid fermentation . In higher animals, the LDH secreted by cells also accumulates in the hemolymph and blood due to its great stability. There it can be used as a laboratory parameter that indicates cell damage. The very similar D- lactate dehydrogenase occurs only in lower animals , microorganisms and plants .
biochemistry

The reduction of pyruvate to lactate by LDH and the cofactor NADH is a characteristic of lactic acid fermentation , in which energy is obtained from glucose without consuming oxygen. The lactate formation serves the purpose of regenerating NAD + , which is required in the glycolysis of GAPDH . Lactate itself can no longer be used in the fermentation process and is excreted by the cells as an end product. In humans and higher animals, lactate is transported to the liver via the bloodstream . The reverse reaction takes place there in the presence of NAD + . Lactate is oxidized to pyruvate and is therefore accessible again to the metabolism. The reaction also occurs in some muscle tissues if NAD + is available.
The reduction of pyruvate to lactate is strongly exergonic (ΔG 0 '= -25 kJ / mol).
Isoforms
Various isoenzymes of LDH are found in the human body . The LDH always consists of four subunits.
The classic are the five forms LDH-1 to LDH-5, which are made up of the subunits LDH-H and / or LDH-M. There are five possible combinations, depending on the type of composition of these two types. The isoenzymes predominant in the respective organ are given below:
- LDH-1 (4H) - in the heart , erythrocytes and kidney
- LDH-2 (3H1M) - found in red blood cells, kidneys, heart and lungs
- LDH-3 (2H2M) - in the cells of the lungs , platelets and lymphatic system
- LDH-4 (1H3M) - in various organs
- LDH-5 (4M) - within the liver and striated muscles
It should be noted that other LDH isoenzymes also occur in the organs mentioned, so LDH-2 is also found in the heart (and traces of LDH-3); LDH-1 is the predominant form.
Since the identification of the classic LDH subunits in the 1950s, further LDH isoenzymes have been discovered that are also available as tetramers: LDHC was discovered in the 1960s. It is mainly composed of LDHC subunits and is only expressed in the testes. LDHBx was discovered in the 2010s. LDHBx is a peroxisome -specific subunit. LDHBx is produced during translation of the LDHB mRNA , but the stop codon is interpreted as an amino acid coding codon . Therefore translation continues until the next stop codon. This means that the LDHBx is seven amino acids longer than the classic LDH-H (LDHB) protein. The resulting extension contains a peroxisomal signal sequence so that LDHBx is imported into the peroxisome.
Laboratory diagnostics
The lactate dehydrogenase (LDH) is used as a diagnostic parameter to prove to increased damage of cells. Lysing cells release the enzyme. Due to its great stability, LDH remains in the extracellular space and accumulates in the blood in the event of pathological cell damage. The LDH activity is measured in this or in the plasma / serum. Under physiological conditions the serum activity of the LDH is up to 240 U / l. If it rises above this value, this is due to increased disintegration of cells. Based on the determination of the LDH isoenzymes, it is also possible to understand which organ is responsible for the origin of the increase in enzyme. All isoforms of LDH can be detected within the blood serum, with LDH-1 and -2 predominating. An increase in LDH-1 and LDH-2 can indicate increased cell death of heart muscle cells or blood cells, whereas an increase in LDH-5 would suggest that there is liver damage. Ultimately, a diagnosis can only be made by looking at several findings together.
Especially in the case of haematological diseases, muscle diseases and liver and biliary tract diseases , a laboratory diagnostic LDH test can provide information about the damage that has occurred. In the past, the LDH activity was also determined in heart muscle diseases, which is no longer absolutely necessary thanks to newer measurement parameters ( cardiac troponin ), e.g. In some cases, however, it is carried out, especially when it comes to the detection of a heart attack that is no longer fresh. In comparison to other heart muscle-specific enzymes or enzyme constellations, LDH-1 and LDH-2 remain detectable for a relatively long time. Any cell damage that has occurred can still be detected after up to 20 days.
Reference range
Reference range for measurements at 37 ° C according to IFCCC
- All: <245 U / l
interpretation
Since the LDH occurs in all tissues , in the case of increased values, initially only the statement can be made that tissue damage has occurred.
Most often, elevated values occur in connection with the following diseases:
- the digestive tract (e.g. post-hepatic jaundice due to disorders in the gallbladder or duodenum ),
- the liver ( hepatopathies ; e.g. hepatocellular jaundice ),
- of the heart ( cardiomyopathies ),
- the skeletal muscles (e.g. myositis ),
- the lungs ( vasculopathies ; pulmonary embolism ),
- the kidneys ( nephropathies ; e.g. kidney infarction ),
- of the blood ( anemia ; e.g. haemolysis ),
- the endocrine system ( endocrinopathies ; e.g. hypothyroidism or thyroiditis ),
- Systemic diseases (e.g. carcinoma or sarcoidosis , connectivitis or mononucleosis ).
Lower values can be found in connection with previous radiotherapy .
Due to the ubiquitous occurrence of LDH, this enzyme and its isoforms are not organ-specific, so it is not used as a topographical key enzyme in diagnostics.
Causes of failure
LDH is abundant in red blood cells ( erythrocytes ). For this reason, hemolytic blood samples may show false positive test results, as this hemolysis can also be caused in vitro by incorrect storage, incorrect collection or long transport of the samples.
Importance in research
In the life sciences and in pharmacology , extracellular LDH is used as a marker for cell damage that has occurred , for example in toxicological studies. LDH released into the medium can be detected, among other things, by its reaction with tetrazolium salts to form formazan dyes.
literature
- B. Neumeister, I. Besenthal, H. Liebich, O. Böhm: Clinical guidelines for laboratory diagnostics. Urban & Fischer, Munich / Jena 2003, ISBN 3-437-22231-7 .
- Lothar Thomas: Laboratory and Diagnosis. TH-Books, Frankfurt am Main 2005, ISBN 3-9805215-5-9 .
Web links
- LDH on ExPASy.org
- David Goodsell: Molecule of the Month: Lactate Dehydrogenase
Individual evidence
- ↑ BRENDA - Information on EC 1.1.1.28 - D-lactate dehydrogenase .
- ↑ F. Schueren, T. Lingner, R. George, J. Hofhuis, J. Gartner, S. Thoms: Peroxisomal lactate dehydrogenase is generated by translational readthrough in mammals . In: eLife . tape 3 , 2014, p. e03640 , doi : 10.7554 / eLife.03640 , PMID 25247702 .
- ^ IFCC
- ^ Philippe Furger: 'Labor quick' - laboratory values and laboratory results from A to Z, differential diagnosis, laboratory medicine. 2nd Edition. Thieme 2014, ISBN 978-3-13-147522-0 , p. 87.