Lefamulin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
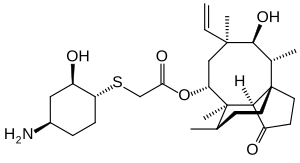
|
||||||||||||||||
| General | ||||||||||||||||
| Non-proprietary name | Lefamulin | |||||||||||||||
| other names |
(3a S , 4 R , 5 S , 6 S , 8 R , 9 R , 9a R , 10 R ) -6-ethenyl-5-hydroxy-4,6,9,10-tetramethyl-1-oxodecahydro-3a, 9-propanocyclopenta [8] annulen-8-yl [((1 R , 2 R , 4 R ) -4-amino-2-hydroxycyclohexyl) sulfanyl] acetate ( IUPAC ) |
|||||||||||||||
| Molecular formula | C 28 H 45 NO 5 S | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| Drug class | ||||||||||||||||
| Mechanism of action |
Elongation phase inhibition |
|||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 507.73 g · mol -1 | |||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lefamulin is an antibiotic active substance from the group of pleuromutilins . In the US, there has been August 2019 as Xenleta for treating community-acquired pneumonia ( Community-Acquired Bacterial Pneumonia admitted CABP).
properties
Lefamulin is a semi-synthetically manufactured substance that is used medicinally as lefamulin acetate and can be given both orally and intravenously .
According to the manufacturer, lefamulin is the first systemically effective antibiotic for human medicine from the group of pleuromutilins. This group of natural substances originating from mushrooms ( Clitopilus passeckerianus , "cat ear rasp ") was discovered in the 1950s. From the lead compound pleuromutilin derived also formerly of skin infections topically used except Lefamulin retapamulin and the active ingredients used in veterinary medicine tiamulin and valnemulin from.
The effect is based on an inhibition of bacterial protein synthesis by lefamulin in the 50S subunit of the ribosomes binding to the peptidyl transferase center located in domain V of the 23S rRNA . The mechanism differs from other antibiotics that target bacterial ribosomes. It is expected that lefamulin will therefore be effective even if resistance has already developed .
Spectrum of activity
In addition to the activity against gram-positive germs ( Streptococcus pneumoniae , Staphylococcus aureus ), the spectrum of activity also includes gram- negative bacteria such as Haemophilus influenzae , Legionella pneumophila , Mycoplasma pneumoniae and Chlamydophila pneumoniae . Lefamulin is not effective against enterobacteria and Pseudomonas aeruginosa .
Clinical information
The approval is based on the results of the two phase III studies LEAP1 and LEAP2 with a total of 1289 patients with community-acquired pneumonia. Lefamulin was compared to the antibiotic moxifloxacin in combination with or without linezolid . In LEAP1 the active ingredients were administered intravenously and in LEAP2 orally. In both studies, lefamulin achieved a clinical success rate similar to that of the comparator therapy.
The most common side effects included diarrhea, nausea, injection site reactions, increased liver enzymes and vomiting. Lefamulin can lead to a prolongation of the QT interval , which increases the risk of life-threatening cardiac arrhythmias . Fetal damage was also observed in animal experiments.
Trade names
Nabriva Therapeutics: Xenleta (USA)
Individual evidence
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ External identifiers or database links for lefamulin acetate : CAS number: 1350636-82-6, EC number: 695-920-8, ECHA InfoCard: 100.224.747 , PubChem : 86346053 , ChemSpider : 34980596 , Wikidata : Q27279882 .
- ^ A b MP Veve, JL Wagner: Lefamulin: Review of a Promising Novel Pleuromutilin Antibiotic . Pharmacotherapy. 38 (2018), Vol. 9, pp. 935-946. doi : 10.1002 / phar.2166 .
- ↑ a b c Prescribing Information Xenleta, August 2019. (PDF; 612 kB) Nabriva Therapeutics US, Inc. (English).
- ↑ a b K. Gräfe: US approval for new antibiotic , Pharmazeutische Zeitung, August 22, 2019.
- ↑ TM File et al .: Efficacy and Safety of Intravenous-to-oral Lefamulin, a Pleuromutilin Antibiotic, for the Treatment of Community-acquired Bacterial Pneumonia: The Phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) Trial . Clinical Infectious Diseases, February 2019. doi : 10.1093 / cid / ciz090 .
- ↑ E. Alexander et al .: Oral Lefamulin vs Moxifloxacin for Early Clinical Response Among Adults With Community-Acquired Bacterial Pneumonia: The LEAP 2 Randomized Clinical Trial . JAMA , September 2019. doi : 10.1001 / jama.2019.15468 .
- ↑ C. Möthrath: FDA grants approval for Xenleta , apotheke adhoc, August 22, 2019.
- ↑ Lefamulin: Novel antibiotic approved in the USA , aerzteblatt.de, August 20, 2019.