Main histocompatibility complex
The major histocompatibility complex or major histocompatibility complex (abbr. MHC of Engl. Major histocompatibility complex ) comprising a group of genes in vertebrates, the proteins encode, which for immune recognition are important, the tissue compatibility (histocompatibility) in transplantation and immunological individuality. MHC regions are found in all vertebrates from cartilaginous fish (sharks, rays). In humans, these genes are mostly found on the short arm of chromosome 6 . The gene products, the MHC protein complexes , are the body's own antigens on the surface of body cells (e.g. easily detectable on white blood cells) and are used to identify the body's own cells. This is where the name HLA system ( Human Leucocyte Antigen ) comes from for the regulatory system of the human immune system, the most important part of which is the MHC. Among other things, the main histocompatibility complex encodes the MHC class I and MHC class II protein complexes, which play a central role in the function of the immune system. In order for antigens to be recognized by T lymphocytes , they must first be prepared and presented on the cell surface on the MHC-coded class I and class II receptors . This phenomenon is called MHC restriction .
The three classes of MHC complexes
Via the MHC class I route, infected and degenerate cells that produce foreign proteins are specifically identified by T killer cells (CD8 + ) and then eliminated. Via the MHC class II pathway, T helper cells (CD4 + ) can stimulate the production of specific antibodies and the activity of phagocytes which inactivate and eliminate pathogens in body fluids (humoral immune response). The adaptive immune system separates intra- and extracellular pathogens. The MHC class III complexes are components of the so-called complement system , a part of the unspecific humoral immune system that contributes to the elimination of cellular antigens (e.g. bacteria). The heavy chain of the MHC class I complexes and the α and β subunits of the MHC class II complexes occur in a large number of alleles in humans (genetic polymorphism ). This is decisive for the importance of the main histocompatibility complex in tissue compatibility.
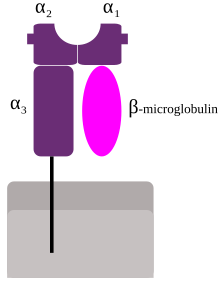
MHC class I complex
These protein complexes are found on the surface of almost all (nucleated) cells of the organism (except trophoblasts and erythrocytes (nucleated) ) and serve to present antigens for cytotoxic T cells and to protect healthy cells from being destroyed by killer cells (→ missing-self hypothesis ) such as T cells or natural killer cells ( NK ). The MHC class I protein complex consists of a larger subunit anchored to the cell membrane; the heavy chain (HC) and a smaller soluble subunit, the β 2 -microglobulin (β 2 M), and an antigenic peptide . In the protein complex , four blank domains distinguished: The α-subunit illustrates three domains (α 1 to α 3 ), the β 2 -microglobulin, the fourth domain. The domains (α 1 and α 2 ) form a pit in which the peptide is bound. These peptides are formed in large numbers and in variety by the proteasome of proteins continuously synthesized in the cytoplasm and are cleavage products of them. The immune system continuously monitors the body for the presence of viral infections and degenerated cells by checking whether cells are endogenous or foreign proteins present and for the presence of MHC-I protein complexes. The presented peptides represent an image of the proteins synthesized in the cells. The cytotoxic T-lymphocytes ( CD8 + T-cells) are selected in such a way that their T-cell receptor usually does not bind to cells that contain a peptide present, which comes from an endogenous protein. This phenomenon is called self-tolerance and protects the body from attacks by its own immune system. However, if a cell is infected with viruses or affected by mutations and thus expresses novel proteins, exogenous peptides are presented to the immune system as part of the MHC class I complex and cytotoxic T lymphocytes are activated, which destroy the affected cells.
MHC class I peptide loading
MHC class I complexes bind peptides 8 to 10 amino acids in length in the peptide binding column. These cannot protrude beyond the gap - the gap is limited at the ends at conserved positions by hydrogen bonds - and are associated with the invariant areas of the gap via the free amino terminus and the free carboxyl terminus . Since the peptides must have a hydrophobic or basic character for stable binding, corresponding subunits of the proteasome are additionally coded on the MHC gene locus , which ensure that peptides with these characteristics are split off more frequently. This ensures that all cytosolic proteins (both endogenous and viral) can be split into MHC-I suitable peptides and presented there. The assembly of the MHC-I takes place in the endoplasmic reticulum (ER) while the proteins in the cytosol are cleaved. The peptide-binding cleft forms in the ER and is never exposed to the cytosol, so the cytosolic peptides must be transported into the ER. Since the MHC-I is unstable without a bound peptide, peptide transport and folding of the MHC-I are closely linked. Newly synthesized α-subunits of MHC-I, which are transported into the ER, bind to the chaperone protein calnexin , which keeps this subunit in a partially folded state. The oxidoreductase ERp57 is probably also part of this complex. If β 2 -microglobulin (β 2 M) binds to this complex, the MHC-I-α-β 2 M complex dissociates from the calnexin and is embedded in a new complex, the peptide loading complex (PLC, peptide loading complex ). Further components of the PLC are the chaperone calreticulin (similar to calnexin, but not membrane-bound), the PLC-specialized chaperone tapasin , ERp57 and the peptide transporter TAP ( transporter associated with antigen presentation ). Calnexin and calreticulin both bind the MHC-I via its sugar chain at asparagine residue 86. The P-domain of calreticulin also binds to ERp57. Tapasin binds covalently to ERp57 and also functions as a link to TAP. The cytosolic peptides are transported into the ER via TAP and attach to MHC-I. If the bond is stable enough, the MHC-I folds completely and the PLC dissociates. The resulting MHC-I peptide complex is transported to the surface in vesicles of the secretory system.
MHC class II complex
MHC class II complexes are professional by specialized cells of the immune system known as antigen presenting cells ( antigen presenting cells APC), and present (of T-helper cells CD4 + cells T) detected. To the APCs include the monocytes and macrophages , interdigitating dendritic cells in Thymusmark, dendritic cells in blood, lymph , epidermis, and other tissues, phagozytoseaktive cells of the vascular endothelium, not phagocytic follicular dendritic cells in secondary follicles of lymph nodes and spleen (but do not express MHC -II) and B lymphocytes . The MHC class II protein complex consists of two membrane-anchored subunits of about the same size, the α and β subunits, and also a peptide. In contrast to the class I complex, here both subunits are anchored in the cell membrane. The protein complex consists of four extracellular domains (α 1 and α 2 as well as β 1 and β 2 ). This complex also forms a pit which is formed by the α 1 and β 1 domains and in which the peptide is bound. The peptides that are presented on MHC class II complexes are derived from extracellular proteins, e.g. B. through receptor-mediated endocytosis, unspecific macropinocytosis or phagocytosis have found access to the secretory pathway of APC. Like the T killer cells, the T helper cells are selected in such a way that they only bind to an MHC class II complex with their T cell receptor and are thus activated when a foreign antigen is presented.
The extracellular proteins are taken up in endosomes by endocytosis . These are acidified in the cell, for example by fusion with a lysosome. The endosomes or lysosomes contain proteases that are activated at low pH. Examples of such proteases are cathepsin B, D, S and L. They break down the proteins into peptides.
Like many other surface proteins, the MHC class II complexes first move into the endoplasmic reticulum. To prevent them from binding to one of the many proteins found in the endoplasmic reticulum, the MHC class II complexes are associated with a protein called the invariant chain. Part of the invariant chain lies in the binding pocket and prevents the binding of other proteins. The invariant chain forms a homotrimer with two others. Until this stable state is reached, the MHC class II complexes are bound to calnexin.
After the trimer is bound, the MHC class II complexes leave the ER. The intracellular compartment with the MHC class II complex fused with an endosome with low pH. The invariant chain is split several times by the proteases. After two cleavages, only a short peptide remains, which is in the binding pocket. This is called CLIP (class II-associated invariant chain peptide) and also prevents the binding of a peptide. To be able to bind a peptide from the endosome, the CLIP has to dissociate or split off. This process is catalyzed by another MHC class II complex, called HLA-DM in humans. It catalyzes the cleavage of the CLIP, binds to empty MHC class II complexes and stabilizes them. HLA-DM itself does not bind peptides, the binding pocket is closed in this complex. In addition, HLA-DM catalyzes the exchange of a weakly bound peptide by one with a higher affinity for the binding pit. Like MHC-I complexes, MHC-II complexes without a bound peptide are unstable and are quickly broken down by proteases. In uninfected cells, they also present the cell's own peptides on the surface.
MHC class III complexes
The class III complexes include the complement factors C2, C4 and Bf, as well as various cytokines , such as tumor necrosis factor (TNF). In contrast to the other classes, these are plasma proteins that are involved in the unspecific immune defense.
literature
- Charles Janeway , Paul Travers, Mark Walport, Mark Shlomchik: Immunology. 5th edition, Spektrum Akademischer Verlag, Heidelberg 2002, ISBN 3-8274-1079-7 ; Online version in English , 5th edition, 2001.
Web links
- Jennifer McDowall / Interpro: Protein Of The Month: Major histocompatibility complex. (engl.)
- Chapter on the generation of MHC-I peptide molecules from the online version of the immunology standard work by Charles Janeway
Individual evidence
- ↑ F. Calabi and C. Milstein: A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. nature volume 323, pp. 540-543, October 9, 1986 doi : 10.1038 / 323540a0
- ↑ Hans-Gustaf Ljunggren and Klas Kärre: In search of the 'missing self': MHC molecules and NK cell recognition. Immunology Today, Vol. 11, pp. 237-244, 1990 doi : 10.1016 / 0167-5699 (90) 90097-S