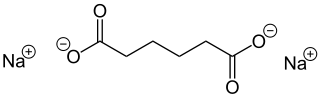
Sodium adipate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium adipate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 8 Na 2 O 4 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 190.11 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| solubility |
584.9 g l −1 (14 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium adipate is a chemical compound from the group of carboxylic acid salts and the sodium salt of adipic acid .
Manufacturing
Sodium adipate can be prepared by reaction of adipic acid with sodium carbonate can be produced.
The neutralization of sodium hydroxide solution with an ethanolic solution of adipic acid also yields sodium adipate.
properties
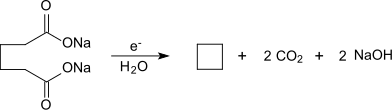
Sodium adipate is a colorless solid that is easily soluble in water. It crystallizes as a hemihydrate in shiny, slightly hygroscopic leaflets. The enthalpy of solution of sodium adipate at 298.15 K is 15.70 kJ mol −1 . The electrolysis of an aqueous solution of sodium adipate produces cyclobutane .
use
Sodium adipate is used as a food additive as a complexing agent and acid regulator . It is also used as a stabilizer for PVC . Barium adipate can be produced by precipitation of a hot barium chloride solution with sodium adipate .
Individual evidence
- ↑ Entry on E 356: Sodium adipate in the European database on food additives, accessed on June 28, 2020.
- ↑ a b c d Entry on disodium adipate in the GESTIS substance database of the IFA , accessed on May 22, 2017(JavaScript required) .
- ^ A b c d W. Dieterle, C. Hell: On the knowledge of adipic acid . In: Ber. d. German chem. Ges . tape 17 , 1884, p. 2221–2228 ( digitized on Gallica ).
- ↑ M. Vera, L. Franco, J. Puiggalí: Synthesis of poly (ester amide) s with lateral groups from a bulk polycondensation reaction with formation of sodium chloride salts . In: Journal of Polymer Science A: Polymer Chemistry 2008 , 46 (2), pp. 661-667. doi: 10.1002 / pola.22414
- ↑ a b Source: Page no longer available , search in web archives: PRODUCT SPECIFICATION Sodium Adipate ( page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.
- ↑ MZH Rozaini, P. Brimblecombe: The solubility measurements of sodium dicarboxylate salts; sodium oxalate, malonate, succinate, glutarate, adipate and in water from T = (279.15 to 358.15) K . In: The Journal of Chemical Thermodynamics 2009 , 41 (9), pp. 980-983. doi: 10.1016 / j.jct.2009.03.017
- ^ Rachna Sagar: Together with Aieee Chemistry , Verlag Rachna Sagar, ISBN 978-81-8137-365-6 , p. 481 ( limited preview in Google Book Search).