Sodium hydroxymethanesulfinate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
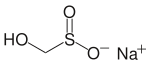
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Sodium hydroxymethanesulfinate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | CH 3 NaO 3 S | ||||||||||||||||||
| Brief description |
white solid with a sulfur-like odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 118.08 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.744 g cm −3 |
||||||||||||||||||
| Melting point |
63 ° C (dihydrate) |
||||||||||||||||||
| solubility |
easily soluble in water (1000 g l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Sodium hydroxymethanesulfinate is an inorganic chemical compound from the group of substituted sulfinic acid derivatives and alcohols . The compound is on the EU's list of CoRAP substances .
Extraction and presentation
Sodium hydroxymethanesulfinate can be obtained by reacting sodium dithionite with formaldehyde in the presence of alkali , by catalytic reduction of sodium hydroxymethanesulfonate with hydrogen and by a number of other preparation methods.
properties
Sodium hydroxymethanesulfinate is a flammable, difficult to ignite, hygroscopic, white solid with a sulfur-like odor, which is easily soluble in water. It decomposes above 125 ° C, producing methanethiol , hydrogen sulfide , some formaldehyde and sulfur dioxide . It is odorless after production, but quickly develops its characteristic odor. The dihydrate gives off its water of crystallization at 120 ° C shortly before the decomposition temperature .
use
Sodium hydroxymethanesulfinate was brought onto the market by BASF in 1905 as "Rongalit C" for the discharge printing of cotton and for vat dyeing . In pharmacy, the compound is used as a preservative, antioxidant and stabilizer in drugs.
Risk assessment
Sodium hydroxymethanesulfinate was included by the EU in 2016 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation in the Community's rolling action plan ( CoRAP ). The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Ingestion of sodium hydroxymethanesulfinate was caused by concerns about worker exposure , high (aggregated) tonnage and widespread use, as well as the possible dangers of carcinogenic, mutagenic and reproductive toxicity properties. The re-evaluation is to be carried out by the Netherlands from 2021 .
Individual evidence
- ↑ a b c d e f g h i Entry on sodium hydroxymethanesulfinate in the GESTIS substance database of the IFA , accessed on January 9, 2019(JavaScript required) .
- ↑ a b c Franz v. Bruchhausen, Siegfried Ebel Eberhard Hackenthal, Ulrike wooden grave: Hager's Handbook of Pharmaceutical Practice sequel 5: fabrics L-Z . Springer-Verlag, 2013, ISBN 978-3-642-58388-9 , pp. 275 ( limited preview in Google Book Search).
- ↑ R. Haller: Chemical Technology of Cotton / Mechanical Aids for Finishing Cotton Textiles 3rd Part . Springer-Verlag, 2013, ISBN 978-3-642-90897-2 , p. 210 ( limited preview in Google Book search).
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): Sodium hydroxymethanesulphinate , accessed on March 26, 2019.

