New World screw worm fly
| New World screw worm fly | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|

New World screwworm fly ( Cochliomyia hominivorax ) |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Cochliomyia hominivorax | ||||||||||||
| ( Coquerel , 1858) |
The New World screw worm fly ( Cochliomyia hominivorax ) is a blowfly species whose maggots attack their host from the outside and thus live as ectoparasites . As so-called obligatory parasites, they always attack warm-blooded animals and feed on their body tissue. This distinguishes them from the larvae of the other three species of the genus Cochliomyia , which, as facultative parasites, only occasionally attack living organisms and otherwise develop in carrion or feces. For this reason, the New World screw worm fly is often referred to as the “primary screw worm fly”. Eggs are laid with preference on wounds, whereupon the larvae hatch and eat under the skin. This infestation can have serious, sometimes fatal consequences for the host organism.
The New World screw worm fly was the first species on which the sterile insect technique was tested and applied in a natural environment. It has been fought and systematically eradicated in North and Central America since the 1950s. It is still widespread in tropical to subtropical parts of Central and South America. Since it is feared that they will be reintroduced via livestock transport, in the USA, for example, infected animal populations must continue to be reported.
description
Imago
The 8 to 10 millimeter tall adults have a metallic, shimmering, blue-green-black colored body, the color of the head and eyes ranges from yellow to orange-reddish and brown. There are three dark, longitudinal stripes on the top of the thorax . They wear black setae on their head shield . These setae distinguish themselves from the similar-looking, sympatric species Cochliomyia macellaria , which has few yellowish setae. The New World screw worm fly is also similar to the Old World screw worm fly ( Chrysomya bezziana ). The most noticeable difference in the females is the color of the Tegula : in the New World screwworm fly it is dark brown-black, in the Old World screwworm fly it is yellow.
There is a sexual dimorphism , in males the eyes are directly or almost directly adjacent ( holoptic or subholoptic), in females the eyes are clearly separated ( dichoptic ). The males also have 12 percent larger wings, but they are more narrowly shaped. In addition, the males are larger. This could be due to the territorial and aggressive behavior towards conspecifics as well as towards alien, mostly smaller insects. The behavior of the female, which only copulates with one male, has an additional effect on competition. The geographic variation has not yet been adequately researched.
larva
The cone-shaped larvae have licking-sucking mouthparts ( mandibles ) at the narrow end . They differ from other fly larvae primarily in their dark, unique trachea . These are recognizable as black stripes and from the third stage onwards run lengthways through the body from the twelfth to the ninth segment. At the broad end, the larva has two breath openings ( stigmas ), which are divided into two slits in the second stage and three slits in the third stage. The stigmas at the narrow end flow into six to eleven, usually seven to nine “twigs” (also called lobi ). Third instar larvae are 6 to 17 millimeters in size - on average 15 millimeters - with a diameter of 1.1 to 3.6 millimeters. Young larvae are creamy white in color. Adult larvae develop a reddish-pink skin. The conspicuous rings of spines that stretch across the body serve the larvae to hold their position in the tissue. The spines can end in one or two pointed ends and are an average of 130 micrometers long in the third larval stage.
Development, way of life and ecology
New World screwworm flies depend on a host ( obligatory parasitism ) because the larvae need living tissue for food. The species is therefore also called "Primary Screwworm Fly" in English. With this way of life it differs from facultative myiasis pathogens ( facultative parasitism ), which mainly utilize necrotic tissue or excrement. Facultative parasites can be attracted by screwworm fly myiasis and are considered secondary myiasis pathogens, such as Cochliomyia macellaria . This species is also called "Secondary Screwworm Fly" because it is the most common cause of myiasis after the New World screwworm fly.
The species is oviparous , the clutches contain 200 to 500 eggs with a production of 200 to 300 eggs in 15 to 20 minutes. Eggs are laid next to the exposed meat of warm-blooded animals and mostly in the late afternoon to evening so that the eggs are not exposed to excessive, harmful solar radiation. The white eggs are laid close together and all aligned in the same way, which is characteristic of the New World screw worm fly. The females can produce clutches up to four times at intervals of three days. During a lifespan of approximately 31 days, the female can travel up to 300 kilometers, but on average only 40 to 55 kilometers per week. During this time a female lays up to 2800 eggs, during which it feeds on nectar and wound secretions . The adults do not need proteins for their diet, but in the female they increase the production of eggs and their development.
The New World screw worm fly prefers to lay its eggs next to wounds, caused for example by dehorning or castration , but also next to the navel of newborns or insect bites. Healthy areas can also be affected with soft tissue or body orifices, such as the inner corner of the eye (medial canthus) or the perineum . The New World screw worm fly attacks wild animals and domestic animals, and more rarely birds and humans. Cold (poikilothermic) animals or carrion are not attacked.
Twelve to twenty-four hours after egg-laying, the larvae hatch and use extraintestinal digestion - made possible by proteolytic enzymes in saliva - to gregariously subcutaneously (i.e. under the skin) into tissue and muscles. The larvae go through three stages with two moults, which promote the growth rate. Five to seven days after hatching, the larvae drop to the ground to bury and pupate. The pupa develops in the puparium , a dark, hardened protection from the epidermis of the third larval stage. After another three to five days, they hatch as imago , usually at the beginning of the day, to dry their wings in the sun on the ground and to escape diurnal predators. Males can be sexually mature in 24 hours, while in females it takes six to seven days for the ovaries to form. However, the female mates as early as three to four days of age and then lays her eggs four days later. Mating takes place on vegetation. Females copulate only once and keep the male's sperm for life, which has been exploited in the extermination programs with the sterile insect technique. The males, on the other hand, mate up to ten times. They behave shyly and hide in the vegetation until they make out a female.
The larval development and pupation time are temperature-dependent and can be completed in 18 days at an average temperature of 29 ° C, or take two to three months under unfavorable conditions; a life cycle can be completed in 20 days. There is no diapause . The larvae die at long-term temperatures below 8 ° C.
Pathology and vector activity
The larvae cause myiasis , which in this case is also known as screwworm myiasis .
Since the larvae eat their way deep into the flesh and only leave a relatively small opening, an infestation is not always obvious. However, wound secretions can flow out of the wound, contaminate the animal's fur and thus indicate an infestation . The foul smell of the wound is characteristic. Other symptoms typical of myiasis can also be used: poor appetite, weight loss and restlessness of the animal, regular licking of the lesion from the animal, lethargy , weakness and restrained movements. The infected animal separates from the herd and looks for shady places to rest. Bleeding can be severe and the surrounding tissues are tight, edematous, and hot. Persistent infestation results in large, irregular, sack-shaped holes. The host can suffer considerable damage as a result and also die, particularly from secondary infections .
The fly plays a role as a vector : For example, it can transmit the foot-and-mouth disease virus and that of classic swine fever . In order to breed the flies for the sterile insect technique, the breeding medium must be germ-free, which is achieved, for example, with formaldehyde .
habitat
The New World screw worm fly prefers to live in tree-covered, shady, water-rich or damp areas. Open and dry terrain without vegetation is avoided. Optimal temperatures range from 20 to 30 ° C. The species does not survive frost; no wintering takes place in the corresponding locations. The biological and climatic needs of the New World and Old World screwworm flies are similar, and although they are geographically separated, there is nothing to prevent their ranges from overlapping in the future if the species were transported through livestock transport.
The natural density is less than 200 flies per square kilometer. It results from the habitat and the number of suitable host animals , and also from the type of agriculture: Smallholders have better control over their animals than farmers who use large and extensively farmed areas.
distribution
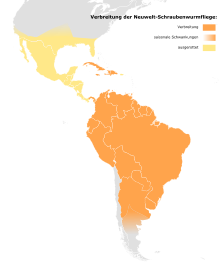
Originally distributed in the southern states of the USA, Mexico , Central America, the northern states of South America to Uruguay and northern Argentina, including some Caribbean islands such as Cuba , Trinidad , Hispaniola , Jamaica and Tobago . Currently, the species is only distributed in South and Central America to the Panama Canal and some Caribbean islands due to the eradication programs. The deposits in southern Argentina are dynamic. They are mainly dependent on the temperature: in winter and early spring, no infestations are recorded in the southern half of Argentina . While the extreme southern and former northern populations collapse in the winter months, they remain stable in the tropics. Before the extinction in the USA, the screwworm fly was able to spread to the Canadian border in favorable years. Spreads happen at a speed of around 80 to 160 kilometers per generation.
Chile is believed to be free of New World screwworm flies, although the exact distribution in the Andes is unknown.
New World screw worm fly and human
Combat
In 1954, the first tests with the sterile insect technology (SIT) were carried out on Curaçao , whereupon the New World screwworm fly was successfully exterminated on the island within four months. From 1958 to 1960 the species was eradicated in the southeastern states of the USA, then in the southwestern states. The species has been considered extinct across the United States since 1966. The fight continued to Mexico (1972–1991), the Virgin Islands (1971–1972), Puerto Rico (1975) and in Central America from Guatemala (1988–1994) to Belize (1988–1994), El Salvador (1991–1995) ), Honduras (1991-1995), Nicaragua (1992-1999) and Costa Rica (1995-2000). In 1998, the first sterile flies were released in Panama. The aim is to build a permanent barrier against New World screwworm fly invasions in Panama. This is achieved through weekly sterile New World screwworm fly releases containing up to 50 million insects. The insects are bred en masse in factories, treated with ionizing radiation and thus sterilized. It is important that the insects bred do not suffer any hindrance to compete with wild, fertile insects. Researchers are investigating the possibilities and methods of producing genetically sterilized males in the future . However, this method is not yet practical.

The fight against it is supported by on-site research: population controls are continuously carried out using fragrance and pheromone traps as well as “meat traps”. Among other things, information on population density and the proportion of sterile and fertile insects can be obtained. The Agricultural Research Service of the USDA studied the behavior, life and distribution via provided with numbers insects. With the research data, a program has already been developed that can simulate population dynamics.
The SIT was not successful in Jamaica between 1999 and 2005 . It is believed that the individuals imported (bred) from Mexico were unable to mate with the native females originally from South America. Before extermination programs are started in South America, further research into copulation compatibility, variations and possible cryptic species of the populations is necessary. The population must also be made aware of future efforts with regard to eradication.
The species was introduced to Libya in 1988 and quickly established and spread there. The SIT was used again: in an area of over 100,000 square kilometers in Libya and neighboring Tunisia , up to 40 million sterile males were released every week. The last case of infected animals dates back to April 1991. However, to be on the safe side, SIT was used for nine further life cycles.
Importance as a disease carrier
People are mainly affected by the neck and head; with more than 100 maggots present in some cases. Serious to fatal lesions resulted from infestations in the ear, mouth, nose and eyes. Healthy people can also be attacked at these body orifices. Human infestation is not uncommon in the areas of distribution. People who work with animals or in fields, especially those with broken skin, are more susceptible to this. In addition, people are at risk who do little personal hygiene, live in poor living conditions or suffer from alcoholism or diabetes . Also sunbathers and the elderly are at increased risk. In Brazil, for example, the New World screwworm fly is responsible for most cases of myiasis. The most common disease associated with myiasis is pediculosis caused by lice , and symptoms of myiasis may be mistaken for pediculosis symptoms by patients.
Although all warm-blooded animals can be used as hosts, the greatest economic losses can be reported in cattle, sheep and goats. In order to reduce the risk of animal infestation, these fluctuations can be exploited in areas where seasonal fluctuations in the New World screwworm fly populations occur. For example, births , castrations, the application of branding , dehorning, ear marking and shearing were planned for New World screwworm-free times. For example, if animals are infected, the California Department of food and agriculture recommends visiting a veterinarian , quarantining the animal, and treating and caring for the wound with a larvicide . Wound care appropriate to the circumstances is of preventive importance. Because larvae of different types and ages can be in a wound, larvae in the deepest part of the wound should be used for diagnostic purposes. The New World screw worm fly cannot be exterminated with wound care alone, since wild animals form a reservoir of hosts.
The damage the New World screwworm fly caused in the southeastern United States between 1958 and 1960 was estimated at $ 20 million, while the eradication program cost $ 11 million. More recent estimates put annual savings for the USA of about 796 million US dollars, for Mexico 292 million US dollars and for Central America around 77.9 million US dollars. The extermination in the southern USA benefited the agricultural and forestry sectors in particular: while in a few years up to 80% of the white-tailed deer offspring fell victim to the New World screwworm fly, the eradication led to an economic boom in deer breeding.
The larva of the New World screw worm fly is not suitable for maggot therapy because of its malignancy .
Systematics and research history
The New World screw worm fly belongs to the genus Cochliomyia of the blowfly family. The species C. aldrichi , C. macellaria and C. minima also belong to the genus .
As Erstbeschreiber applies Charles Coquerel , of 1858 in the annals of Entomologique Société de France the species as Lucilia hominivorax described. The specific epithet hominivorax literally means human eater . The taxonomy within the genus remained controversial for a long time because Coquerel's publication did not reach American entomologists. Furthermore, the similarity of C. macellaria to the New World screw worm fly and their geographical overlap made correct identifications difficult . Because it was mistaken for C. macellaria , infected carcasses were burned or buried in the belief that this would control the New World screwworm fly , until 1933 EC Cushing and PC Patton identified the New World screwworm fly and named it Cochliomyia americana . After identifying the pathogen, Edward F. Knipling and Raymond C. Bushland began to study the species and developed the SIT, for which they received the 1992 World Food Prize .
In older literature the scientific generic name Callitroga can also be found, as in Callitroga hominivorax or Callitroga americana (each indicative of the New World screw worm fly ). The ICZN decided in 1986 that the generic name " Callitroga Brauer, 1883 " was invalid and that " Cochliomyia Townsend, 1915 " should be the official scientific generic name from now on.
swell
literature
- World Organization for Animal Health (OIE) Terrestrial Manual 2008: New World Screwworm (Cochliomyia hominivorax) and Old Worm Srewworm (Chrysomya bezziana) ( Memento of December 9, 2008 in the Internet Archive ) (PDF document, 491.5 KB, English; including drawings and photos). Retrieved October 15, 2010.
- Luis Trombetta et al .: Case Report: Cutaneous myiasis due to Cochliomyia hominivorax in a drug user , (PDF document, 212 KB, English). Copyright © 2009 Trombetta et al. Pp. 874-875. Retrieved October 26, 2010.
- M. Vargas-Terán et al .: Impact of Screwworm Eradication Programs using the Sterile Insect Technique (PDF, 133.5 KB, English). Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, 629–650. © 2005 IAEA. Jumper. Printed in the Netherlands. P. 635. Retrieved November 24, 2010.
- Animal Health Australia: Australian Veterinary Emergency Plan (AUSVETPLAN); Disease Strategy Screw-worm fly, Version 3.0, 2007 , (PDF document, 52.2 KB, English). Retrieved October 2, 2010.
Web links
- Article from the Food and Agriculture Organization (FAO):
- MJR Hall: Screwworm flies as agents of wound myiasis , (English). Retrieved October 28, 2010.
- M. Vargas-Terán: The new world screwworm in Mexico and Central America , (English). Retrieved October 28, 2010.
- James E. Novy: Screwworm control and eradication in the southern United States of America (English). Retrieved November 30, 2010.
- Screwworm Eradication Program Records . A collection of text, audio, visual and video material provided by the National Agricultural Library of the United States Department of Agriculture .
- Cochliomyia hominivorax on merckvetmanual.com (English). Retrieved October 2, 2010.
- Entry in uBio: Cochliomyia hominivorax , (English). Retrieved October 2, 2010.
- Images of larvae and adults , provided by DPDx . Retrieved October 22, 2010.
Individual evidence
- ^ Proceedings of the Entomological Society of Washington . In the Biodiversity Heritage Library . Retrieved October 10, 2010.
- ↑ a b Animal Health Australia: Australian Veterinary Emergency Plan (AUSVETPLAN); Disease Strategy Screw-worm fly, Version 3.0, 2007 , (PDF document, 52.2 KB, English). P. 9. Retrieved October 2, 2010.
- ↑ World Organization for Animal Health (OIE) Terrestrial Manual 2008: New World Screwworm (Cochliomyia hominivorax) and Old Worm Srewworm (Chrysomya bezziana) ( Memento of December 9, 2008 in the Internet Archive ) (PDF, 491.5 KB, English; including drawings and Photos). P. 270. Retrieved October 15, 2010.
- ↑ a b James P. Dear: A Revision of the New World Chrysomyini (Diptera: Calliphoridae) (PDF document, 16.2 MB, English; including drawings). Revista Brasileira de Zoologia, Volume 3 1985. p. 136.
- ↑ ML Lyra et al .: Wing morphometry as a tool for correct identification of primary and secondary New World screwworm fly (PDF document, 16.2 MB, English; including drawings and graphics). Bulletin of Entomological Research (2010). S. 21. Accessed November 22, 2010. doi : 10.1017 / S0007485309006762
- ↑ ML Lyra et al .: Wing morphometry as a tool for correct identification of primary and secondary New World screwworm fly (PDF document, 16.2 MB, English; including drawings and graphics). Bulletin of Entomological Research (2010). P. 23. Retrieved November 22, 2010. doi : 10.1017 / S0007485309006762
- ↑ a b M. L. Lyra et al .: Wing morphometry as a tool for correct identification of primary and secondary New World screwworm fly (PDF document, 16.2 MB, English; including drawings and graphics). Bulletin of Entomological Research (2010). P. 20. Accessed November 22, 2010. doi : 10.1017 / S0007485309006762
- ↑ Thomas Schnieder (Ed.): Veterinary Parasitology . Parey in MVS Medizinverlage Stuttgart GmbH & Co, Stuttgart 2006, ISBN 3-8304-4135-5 ( limited preview in the Google book search).
- ↑ Thomas Schnieder (Ed.): Veterinary Parasitology . Parey in MVS Medizinverlage Stuttgart GmbH & Co, Stuttgart 2006, ISBN 3-8304-4135-5 ( limited preview in the Google book search).
- ↑ World Organization for Animal Health (OIE) Terrestrial Manual 2008: New World Screwworm (Cochliomyia hominivorax) and Old Worm Srewworm (Chrysomya bezziana) ( Memento of December 9, 2008 in the Internet Archive ) (PDF document, 491.5 KB, English; included Drawings and photos). P. 269. Retrieved October 15, 2010.
- ↑ a b M. JR Hall: Screwworm flies as agents of wound myiasis , (English). Article from the Food and Agriculture Organization (FAO). Retrieved October 28, 2010.
- ↑ John G. Riemann: The Development of Eggs of Cochliomyia Hominivorax (Coquerel) , (English). Retrieved October 9, 2010.
- ↑ a b California Department of Food and Agriculture Animal Health Branch: Fact Sheet: Screwworm (PDF document, 52.2 KB, English). Retrieved October 2, 2010.
- ↑ a b Animal Health Australia: Australian Veterinary Emergency Plan (AUSVETPLAN); Disease Strategy Screw-worm fly, Version 3.0, 2007 , (PDF document, 52.2 KB, English). P. 16. Retrieved October 2, 2010.
- ↑ a b Graham B. White et al .: Filth Flies: Significance, Surveillance and Control in Contingency Operations ( Memento of the original from March 3, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (English). Published by the Armed Forces Pest Management Board. Retrieved October 17, 2010.
- ^ Animal Health Australia: Australian Veterinary Emergency Plan (AUSVETPLAN); Disease Strategy Screw-worm fly, Version 3.0, 2007 , (PDF document, 52.2 KB, English). S. 11. Retrieved October 2, 2010.
- ^ Heinrich Behrens et al .: Textbook of Sheep Diseases . Blackwell Wissenschafts-Verlag, Berlin, Vienna 2001, ISBN 3-8263-3186-9 ( limited preview in Google book search).
- ↑ World Organization for Animal Health (OIE) Terrestrial Manual 2008: New World Screwworm (Cochliomyia hominivorax) and Old Worm Srewworm (Chrysomya bezziana) ( Memento of December 9, 2008 in the Internet Archive ), (PDF document, 491.5 KB, English; including drawings and photos). Retrieved October 15, 2010.
- ↑ a b World Organization for Animal Health (OIE) Terrestrial Manual 2008: New World Screwworm (Cochliomyia hominivorax) and Old Worm Srewworm (Chrysomya bezziana) ( Memento of December 9, 2008 in the Internet Archive ) (PDF, 491.5 KB, English; included Drawings and photos). Retrieved October 15, 2010.
- ↑ a b International Screwworm Program ( Memento of the original from October 7, 2010 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (English). United States Department of Agriculture (USDA). Retrieved October 8, 2010.
- ^ M. Donald Mc Cavin et al .: Pathology of Domestic Animals . 1st edition. Urban & Fischer Verlag , Stuttgart 2009, ISBN 978-3-437-58250-9 , pp. 1077 ( limited preview in Google Book search).
- ↑ Cochliomyia hominivorax on merckvetmanual.com. Retrieved October 2, 2010.
- ↑ a b Animal Health Australia: Australian Veterinary Emergency Plan (AUSVETPLAN); Disease Strategy Screw-worm fly, Version 3.0, 2007 , (PDF document, 52.2 KB, English). P. 12. Accessed October 2, 2010.
- ↑ MF Chaudhury et al .: Screwworms, Cochliomyia hominivorax, reared for mass release do not carry and spread foot-and-mouth disease virus and classical swine fever virus , (PDF document, 162.9 KB, English). Journal of Insect Science: Vol. 8 | Article 62, published October 23, 2008. Retrieved October 23, 2010.
- ↑ World Organization for Animal Health (OIE) Terrestrial Manual 2008: New World Screwworm (Cochliomyia hominivorax) and Old Worm Srewworm (Chrysomya bezziana) ( Memento of December 9, 2008 in the Internet Archive ) (PDF, 491.5 KB, English; including drawings and Photos). P. 267. Retrieved October 15, 2010.
- ^ Animal Health Australia: Australian Veterinary Emergency Plan (AUSVETPLAN); Disease Strategy Screw-worm fly, Version 3.0, 2007 , (PDF document, 52.2 KB, English). S. 17. Retrieved October 2, 2010.
- ↑ a b Luis Trombetta et al .: Case Report: Cutaneous myiasis due to Cochliomyia hominivorax in a drug user , (PDF document, 212 KB, English). Copyright © 2009 Trombetta et al. Pp. 874-875. Retrieved October 26, 2010
- ↑ a b c World Organization for Animal Health (OIE) Terrestrial Manual 2008: New World Screwworm (Cochliomyia hominivorax) and Old Worm Srewworm (Chrysomya bezziana) ( Memento of December 9, 2008 in the Internet Archive ) (PDF, 491.5 KB, English; including drawings and photos). P. 266. Retrieved October 15, 2010.
- ↑ a b c James E. Novy: Screwworm control and eradication in the southern United States of America (English). Article from the Food and Agriculture Organization (FAO). Retrieved November 30, 2010.
- ↑ M. Vargas-Terán et al .: Impact of Screwworm Eradication Programs using the Sterile Insect Technique (PDF, 133.5 KB, English). Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, 629-650. 2005 IAEA. Jumper. P. 639. Retrieved November 24, 2010.
- ↑ M. Vargas-Terán et al .: Impact of Screwworm Eradication Programs using the Sterile Insect Technique ( Memento of the original from July 4, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF, 133.5 KB, English). Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, 629-650. © 2005 IAEA. Jumper. Printed in the Netherlands. P. 635. Retrieved November 24, 2010.
- ↑ Margret L. Allen and Phillip L. Scholl: Quality of Transgenic Laboratory Strains of Cochliomyia hominivorax (Diptera: Calliphoridae) . Entomological Society of America, 2005. doi : 10.1603 / 0022-0493-98.6.2301 . P. 2305.
- ↑ Linda McGraw: Squeezing Out Screwworm , (English). April 2001 issue of Agricultural Research magazine . Retrieved December 10, 2010.
- ↑ L. Mc Donagh et al .: Phylogenetic analysis of New World screwworm fly, Cochliomyia hominivorax, suggests genetic isolation of some Caribbean island populations following colonization from South America (English). 2009, The Royal Entomological Society. Retrieved November 23, 2010. doi : 10.1111 / j.1365-2915.2008.00777.x
- ↑ M. Vargas-Terán et al .: Impact of Screwworm Eradication Programs using the Sterile Insect Technique ( Memento of the original from July 4, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF, 133.5 KB, English). Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, 629-650. © 2005 IAEA. Jumper. Printed in the Netherlands. P. 639. Retrieved November 24, 2010.
- ^ British journal of entomology and natural history, p. 160 . In the Biodiversity Heritage Library . Retrieved October 10, 2010.
- Jump up ↑ Screwworm ( Cochliomyia hominivorax ) - a now menace for Africa . In the Biodiversity Heritage Library . Retrieved October 10, 2010.
- ↑ MC Ribeiro, A. d. Pepato, FP De Matos, CE Sverzut, AA Abrahão, AE Trivellato: Oral myiasis in an elderly patient. In: Gerodontology. Volume 29, number 2, June 2012, pp. E1136 – e1139, doi : 10.1111 / j.1741-2358.2010.00432.x , PMID 21029155 .
- ↑ ACP Ferraz et al .: First Record of Human Myiasis Caused by Association of the Species Chrysomya megacephala (Diptera: Calliphoridae), Sarcophaga (Liopygia) ruficornis (Diptera: Sarcophagidae), and Musca domestica (Diptera: Muscidae) (English). Journal of Medical Entomology 47 (3): p. 487, 2010. doi : 10.1603 / ME09143 .
- ↑ M. Vargas-Terán et al .: Impact of Screwworm Eradication Programs using the Sterile Insect Technique ( Memento of the original from July 4, 2009 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF, 133.5 KB, English). Sterile Insect Technique. Principles and Practice in Area-Wide Integrated Pest Management, 629-650. © 2005 IAEA. Jumper. Printed in the Netherlands. P. 630. Retrieved November 24, 2010.
- ↑ Martin Grassberger: Fly Maggots: Parasites and wound healers (PDF; 3.6 MB). Biology Center Linz / Austria. P. 524.
- ↑ Terry Whitworth: Key to the Genera and Species of Blow Flies (Diptera: calliphoridae) of North America of Mexico (PDF document, 27.2 MB, English; including drawings). Proceedings of the Entomological Society of Washington, 2006. pp. 710-713. Retrieved November 14, 2010.
- ↑ Terry Whitworth: Keys to the genera and species of blow flies (Diptera: Calliphoridae) of the West Indies and description of a new species of Lucilia Robineau-Desvoidy (PDF document, 2.1 MB, English; including drawings and photos) . Zootaxa 2663: 1-35 (2010). Retrieved November 21, 2010.
- ↑ Entry in uBio: Cochliomyia hominivorax , (English). Retrieved October 2, 2010.
- ↑ Charles Coquerel: Notes sur des larves appartenant a une nouvelle èspece de diptère, (Cochliomyia hominivorax) (French). Annales de la sociéte entomologique de France, sixth volume, 1858.
- ↑ a b V. A. Dyck et al .: Sterile Insect Technique . Springer, 2005, ISBN 1-4020-4050-4 , pp. 9 ( limited preview in Google Book search).
- ^ The World Food Prize: 1992: Knipling and Bushland . Retrieved January 18, 2011.
- ^ PK Tubbs: Opinion 1399. Cochliomyia Townsend 1915 (Diptera, Calliphoridae): Conserved . (English). The Bulletin of Zoological Nomenclature, Volume 43, 1986. p. 154.