Omethoate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
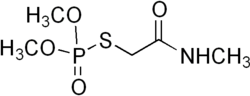
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Omethoate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 12 NO 4 PS | |||||||||||||||
| Brief description |
colorless liquid with a mercaptan-like odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 213.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.32 g cm −3 |
|||||||||||||||
| Melting point |
−28 ° C |
|||||||||||||||
| boiling point |
135 ° C (decomposition) |
|||||||||||||||
| Vapor pressure |
<0.1 Pa |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.4987 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Omethoate is a chemical compound from the group of carboxamides and thiophosphoric acid esters .
Extraction and presentation
Omethoate can be produced by reacting O , O -dimethylphosphoryl mercaptoacetic acid with methyl isocyanate or by reacting O , O -dimethylthiophosphoric acid with 2-chloro- N- methylacetamide .
properties
Omethoat is a flammable, slightly volatile, oily, colorless liquid with a mercaptan- like odor that is miscible with water. It decomposes when heated above 135 ° C. Omethoate hydrolyzes slowly in acidic and more rapidly in basic conditions.
use
Omethoate is used as an insecticide . It is a metabolite and P = O analog (oxon) of dimethoate and works by inhibiting acetylcholinesterase .
Admission
Omethoat was approved in the GDR between 1976 and 1994 and in the FRG between 1971 and 1998.
In 2002 it was not included in the list of permitted active ingredients in plant protection products in Annex I of Directive 91/414 / EEC. In Germany, Austria and Switzerland, no pesticides with this active ingredient are permitted.
Individual evidence
- ↑ a b c d e f g h i j k l Entry on omethoate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b Joint Meeting on Pesticide Residues (JMPR), Monograph for Omethoate , accessed December 9, 2014.
- ↑ Entry on Omethoate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on Omethoat in the Hazardous Substances Data Bank , accessed February 6, 2013.
- ↑ Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-08-095716-1 , pp. 329 ( limited preview in Google Book search).
- ^ A b Terence Robert Roberts: Metabolic Pathways of Agrochemicals: Insecticides and Fungicides . Royal Society of Chemistry, 1999, ISBN 0-85404-499-X , pp. 398 ( limited preview in Google Book search).
- ↑ Peter Brandt (Ed.): Reports on Plant Protection Products 2009: Active Ingredients in Plant Protection Products ; Approval history and regulations of the Plant Protection Application Ordinance . Springer, 2010, ISBN 978-3-0348-0028-0 , pp. 22 ( limited preview in Google Book search).
- ↑ Regulation (EC) No. 2076/2002 of the Commission of November 20, 2002 extending the deadline in accordance with Article 8 (2) of Council Directive 91/414 / EEC and on the non-inclusion of certain active substances in Annex I of this directive as well as the revocation of the Approvals of plant protection products with these active ingredients (PDF)
- ↑ General Directorate Health and Food Safety of the European Commission: Entry on Omethoate in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; accessed on February 24, 2016.

