Levonorgestrel
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
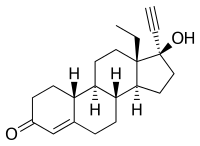
|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Levonorgestrel | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula | C 21 H 28 O 2 | |||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code |
G03 AC03 G03 AA07 G03 AB03 G03 DA06 G03 FA11 G03 FB09 G02 BA04 |
|||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 312.45 g · mol -1 | |||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Levonorgestrel is a synthetic progestogen of the 2nd generation which is used for hormonal birth control (contraception).
application areas
Levonorgestrel was brought onto the market in 1966 and thus belongs to the 2nd generation of synthetic gestagens. As an active ingredient, it is used in various ways for hormonal contraception:
- Birth control pills (in combination with an estrogen derivative )
- Subdermal hormone implant
- Intrauterine device with hormone release (" Hormonspirale ", trade name Mirena )
- Intrauterine drug delivery system
- Mini pill
- Morning-after pill (oral post-coital contraception)
Another area of application is hormone replacement therapy for menopausal symptoms.
effect
The effect of Levonorgestrel depends on the dose and the time of use in relation to the female menstrual cycle . As part of contraception (as part of the birth control pill, mini pill or long-term contraceptives such as the hormone coil), levonorgestrel inhibits the release of so-called gonadotropic hormones from the pituitary gland . The gonadotropic hormones LH and FSH control the activity of the ovaries and the remodeling processes in the uterine lining .
Furthermore, the effect of levonorgestrel makes the secretion of the cervix tougher. This means that sperm cannot reach the uterus, or only very sporadically. Further effects of the Levonorgestrel concern the tubal motility of the fallopian tubes.
The exact effect of Levonorgestrels in the context of emergency contraception (morning-after pill) is unclear. Inhibition of ovulation, an inhibiting effect on the transport of the egg cell or sperm in the fallopian tubes and a disruption of the implantation of the fertilized egg cell are discussed .
Submission and reimbursement of the "morning after pill"
The morning-after pill was subject to prescription in Germany until March 2015 . Although the responsible committee of the Federal Institute for Drugs and Medical Devices (BfArM) had already spoken in 2004 in favor of releasing the morning-after pill with the active ingredient levonorgestrel from the prescription requirement, the dispensing regulation had not been changed.
In May 2012, Pro familia started the “Breakdown assistance after 6” campaign with the aim of making the “morning after pill” prescription-free. In an open letter from November 2012, the professional association of gynecologists (BVF) and the German Society for Gynecology and Obstetrics (DGGG) spoke out against the prescription-free levonorgestrel.
With the release from the prescription requirement on March 15, 2015, the assumption of costs by the health insurance company for patients under 20 years of age (under 22 years of age since March 2019) was also prescribed. Distribution by mail order is not permitted; direct advertising for the product is prohibited. Adolescents aged 14 and over can also acquire levonorgestrel without the consent of their legal guardians . Comprehensive advice to those affected is recommended before purchasing.
In Switzerland, the morning-after pill is available without a prescription after a personal interview at the pharmacy. In Austria, too, levonorgestrel is given for emergency contraception without a prescription, as is the case in many other EU countries, cf. Requirements for levonorgestrel .
Adverse drug effects
The evaluation of side effect reports in the VigiBase database of the Uppsala Monitoring Center of the WHO showed that levonorgestrel as well as other anti-contraceptive active ingredients or active ingredient combinations ( medroxyprogesterone , drospirenone / ethinylestradiol , etonogestrel , ethinylestradiol / ethinylestradiol / etonogestrelel , ethinylestradiol / ethinylestradiol , desogestradinyl , desogestrelel) and desogestrel / ethinylestradiol) has the potential for a complete loss of libido when used systemically . The author concludes that a clear reference to this possible adverse drug effect in the product information is advisable in order to avoid a possible loss of quality of life through a change in the choice of birth control .
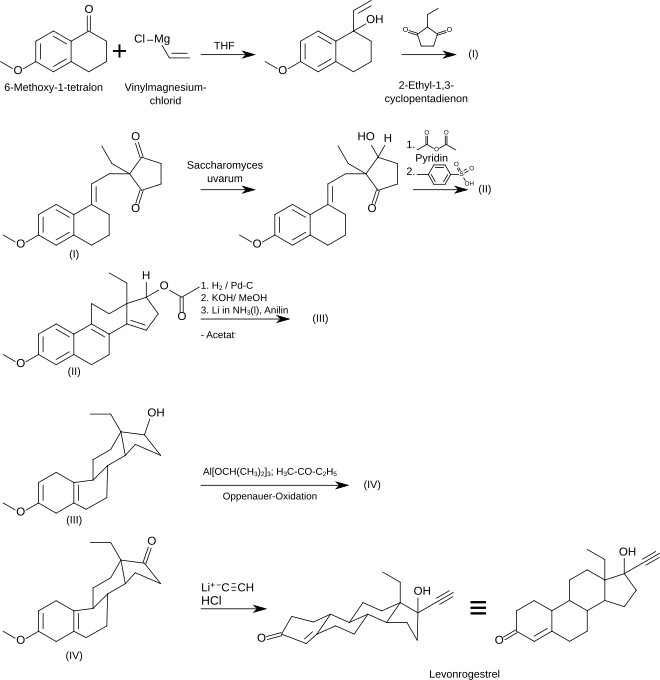
synthesis
A multi-step enantioselective synthesis of levonorgestrel is described in the specialist literature, starting from 6-methoxy-1-tetralone:
Analytics
For reliable qualitative and quantitative determination in various test items such as B. serum or plasma , urine or waste water samples , the coupling of chromatography with mass spectrometry is used after adequate sample preparation . This analytical procedure is also used for the determination in rainbow trout exposed to wastewater .
literature
- Alexander T. Teichmann , Alan Corbin: Levonorgestrel. Thieme, Stuttgart / New York 1998, ISBN 3-13-104721-6 .
- R. Sitruk-Ware: New progestagens for contraceptive use. In: Hum Reprod Update . Volume 12, 2006, pp. 169-178. PMID 16291771 doi: 10.1093 / humupd / dmi046 .
Individual evidence
- ↑ a b data sheet (-) - Norgestrel at Sigma-Aldrich , accessed on April 7, 2011 ( PDF ).
- ↑ morning-after pill * must be available without a prescription! "Breakdown assistance after 6" campaign. In: profamilia.de , May 21, 2012.
- ^ To the members of the German Bundestag - Open letter from BVF and DGGG on the prescription-free levonorgestrel dated November 9, 2012.
- ↑ Federal Council approves the prescription-free morning-after pill . In: Süddeutsche Zeitung . March 6, 2015.
- ^ Celine Müller: Health insurers pay birth control pills up to the age of 22 years. In: DAZ.online. April 5, 2019, accessed June 21, 2019 .
- ↑ Bundestag approves the morning-after pill . In: apotheke-adhoc.de , February 27, 2015.
- ^ Federal Chamber of Pharmacists : Prescription-free dispensing of emergency contraceptives (“morning-after pill”). P. 10. ( Memento of the original from April 2, 2015 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. As of January 28, 2015, accessed February 28, 2015.
- ↑ WHO Pharmaceuticals Newsletter No. 5, 2017
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher, Dieter Reichert: Pharmaceutical Substances. 5th edition. Thieme-Verlag, Stuttgart 2009, ISBN 978-3-13-558405-8 , pp. 799-800; also online with biannual additions and updates.
- ↑ R. Wang, Y. Tian, L. Zhang, Z. Zhang: Simultaneous determination of levonorgestrel and two endogenous sex hormones in human plasma based on LC-MS / MS. In: Bioanalysis. 8 (11), Jun 2016, pp. 1133-1144. PMID 27211854
- ↑ S. Ananthula, DR Janagam, S. Jamalapuram, JR Johnson, TD Mandrell, TL Lowe: Development and validation of sensitive LC / MS / MS method for quantitative bioanalysis of levonorgestrel in rat plasma and application to pharmacokinetics study. In: J Chromatogr B Analyt Technol Biomed Life Sci. 1003, Oct 15, 2015, pp. 47-53. PMID 26409262
- ↑ PG Zanchetta, O. Heringer, R. Scherer, HP Pacheco, R. Gonçalves, A. Pena: Evaluation of storage and evaporation in the removal efficiency of D-norgestrel and progesterone in human urine. In: Environ Monit Assess. 187 (10), Oct 2015, p. 619. PMID 26353967
- ↑ P. Avar, G. Maasz, P. Takács, S. Lovas, Z. Zrinyi, R. Svigruha, A. Takátsy, LG Tóth, Z. Pirger: HPLC-MS / MS analysis of steroid hormones in environmental water samples. In: Drug Test Anal. 8 (1), Jan 2016, pp. 123-127. PMID 26059287
- ↑ FF Al-Qaim, MP Abdullah, MR Othman, J. Latip, Z. Zakaria: Multi-residue analytical methodology-based liquid chromatography-time-of-flight-mass spectrometry for the analysis of pharmaceutical residues in surface water and effluents from sewage treatment plants and hospitals. In: J Chromatogr A. 1345, Jun 6, 2014, pp. 139-153. PMID 24768127
- ↑ J. Fick, RH Lindberg, J. Parkkonen, B. Arvidsson, M. Tysklind, DG Larsson: Therapeutic levels of levonorgestrel detected in blood plasma of fish: results from screening rainbow trout exposed to treated sewage effluents. In: Environ Sci Technol. 44 (7), Apr 1, 2010, pp. 2661-2666. PMID 20222725
Trade names
Levogynon (D), Microlut (D), 28 mini (D), Mirena (D, A, CH), NorLevo (CH), Postinor (A), Unofem (D), Vikela (A)
Asumate (D), CycloÖstrogynal (D), Cyclo-Progynova (D), Fem7 Combi (D, A), Femigoa (D), Femigyne (D), Gravistat (D), Illina (D), Klimonorm (D), Leios (D), Leona (D), Leonore (A), Levomin 20 (D), Loette (A), Madonella (A), Microgynon (D, A, CH), Minisiston (D), Minisiston 20 fem (D ), Miranova (D, CH), MonoStep (D), NovaStep (D), Ologyn (CH), Östronara (D), Rigevidon (A), Selina mite Gynial (A), Swingo (D), Triette (D) , Trigoa (D), Triquilar (D), Triregol (A), Trisiston (D), Wellnara (D), Xyliette (A)