Vinyl propionate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
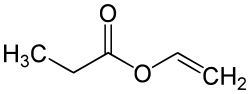
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Vinyl propionate | |||||||||||||||
| other names |
Vinyl propionate |
|||||||||||||||
| Molecular formula | C 5 H 8 O 2 | |||||||||||||||
| Brief description |
highly flammable, volatile, colorless liquid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 100.12 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.92 g cm −3 |
|||||||||||||||
| Melting point |
−81 ° C |
|||||||||||||||
| boiling point |
95 ° C |
|||||||||||||||
| Vapor pressure |
64.5 hPa (20 ° C) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.403 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Vinyl propionate is a chemical compound from the group of propionic acid esters .
Extraction and presentation
Vinyl propionate can be obtained from ethyne and propionic acid in the gas phase with zinc propionate on activated carbon as a catalyst .
properties
Vinyl propionate is a highly flammable, volatile, colorless liquid. It is sparingly soluble in water and has unlimited solubility in ethanol , all common solvents and vegetable oils. It tends to polymerize and is therefore industrially mixed with inhibitors such as 4-methoxyphenol in the range of about 100 ppm.
use
Vinyl propionate is used as a starting material for the production of homopolymers and copolymers with monomers such as (meth) acrylic compounds, vinyl chloride and other vinyl esters .
safety instructions
The vapors of vinyl propionate can form an explosive mixture with air ( flash point 1 ° C, ignition temperature 385 ° C). Like the structurally related vinyl acetate, vinyl propionate is rapidly hydrolytically split by esterases to give the respective acid and vinyl alcohol , which is then further metabolized to acetaldehyde . Acetaldehyde and vinyl acetate are classified in category 3 of the carcinogenic substances.
Individual evidence
- ↑ a b c d e f g h i j k Entry on vinyl propionate in the GESTIS substance database of the IFA , accessed on February 14, 2017(JavaScript required) .
- ↑ a b c d Toxicological assessment of vinyl propionate (PDF) at the professional association for raw materials and chemical industry (BG RCI), accessed on August 22, 2012.
- ↑ a b Datasheet Vinyl propionate from Sigma-Aldrich , accessed on April 25, 2011 ( PDF ).

