Pseudocapacitance
A pseudo-capacity stores electrical energy by means of reversible redox reactions at suitable electrodes of an electrochemical capacitor ( super capacitor ) having a Helmholtz - bilayer . The redox reactions are associated with a Faraday charge exchange from the ions in the electrolyte to the metallically conductive ions in the electrode. Only one electron from a desolvated and adsorbed ion is involved. The adsorbed ion does not form a chemical bond with the electrode. There is only one electron transfer .
A pseudocapacitance only ever occurs together with a double-layer capacitance . In all electrochemical capacitors ( supercapacitors ), they inseparably add up to a total capacitance . However, depending on the design of the electrodes, they have a very different proportion of the total capacitance. The pseudocapacitance of an electrode suitable for this can, for example, be a factor of 100 greater than the double-layer capacitance with the same electrode surface.
The amount of charge of the energy stored in a pseudo capacitance is linear to the applied voltage . The unit of the pseudocapacitance is farad .
history
For the history of the theoretical models for pseudocapacitance see electrochemical double layer .
For the history of the development of electrochemical capacitors, see Supercapacitor .
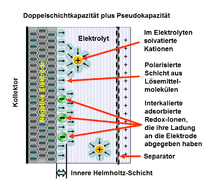
How the electrochemical pseudocapacitance works

1. Inner Helmholtz layer ( English inner Helmholtz plane , IHP),
2. Outer Helmholtz layer ( English outer Helmholtz plane , OHP),
3. Diffuse layer,
4. Solvated cations ,
5. Desolvated and adsorbed anion (redox ion which contributes to the pseudocapacity),
6. Molecules of the electrolyte solvent
Redox reactions with Faraday charge exchange have been known from accumulators for decades. But these chemical processes are connected with strong chemical bonds between the electrode material and an adsorbate from the electrolyte. Although the chemical processes are relatively reversible, the charge / discharge cycles in rechargeable batteries leave behind irreversible chemical compounds that limit the storage capacity and thus the service life. In addition, the chemical reactions in batteries are quite slow, so that charging / discharging takes a longer time.
Pseudocapacitive redox reactions in electrochemical capacitors ( supercapacitors ) are different. They take place with a physical adsorption ( physisorption ) of a charged molecule or atom ( ion ) on the electrode surface and is similar to a chemical equilibrium reaction . However, the adsorbed substance ( adsorbate ) does not form a chemical bond with the surface, but adheres due to weaker forces similar to adhesion . As a rule, only Van der Waals forces occur. The ions to be adsorbed first have to overcome the separating effect of the electrochemical double layer in the supercapacitor. In doing so, they lose the surrounding solvation shell . When the ions are then adsorbed from the electrolyte, a Faraday charge exchange takes place on the surface of the suitable electrode . Only one electron is involved in each redox reaction. There is only one electron transfer (one-electron exchange reaction). In these outer-sphere redox reactions , no bonds are made or broken. This process is reversible, ie when the capacitor is discharged, the electron transfer takes place in the opposite direction.
The ability of capacitor electrodes to bring about redox reactions for a pseudocapacitance depends very much on the nature and structure of the electrode material. Electrode materials that have pseudocapacitive properties are e.g. B. Metal oxides of transition metals , some of which are introduced into the electrode material by doping or inserted with the aid of intercalation . Conductive polymers such as polyaniline or derivatives of polythiophene that are applied to the structures of carbon electrodes are also suitable for pseudocapacitors. But carbon electrodes can also have a pseudocapacitance. The proportion of pseudocapacitive reactions on carbon electrodes can also be significantly increased by tailor-made pore sizes.
There can be three types of electrochemical energy storage with an electron transfer leading to a pseudocapacitance in supercapacitors:
- Redox reactions (reduction-oxidation reactions) with specifically adsorbed ions from the electrolyte on the surfaces of the electrodes
- Intercalation , insertion of atoms into the lattice structure of the electrode
- Electrosorption , underpotential deposition of hydrogen atoms or metallic Ad atoms in surface lattice sites of the electrode lattice structure
Description of the types of systems that contribute to the pseudocapacity:
- Redox system: Ox + ze‾ ⇌ Red and O 2 ‾ + H ˡ ⇌ in the grid
- Intercalation system: Liˡ in "Ma 2 "
- Electrosorption, underpotential deposition of metal adatoms: M꞊ ˡ + S + ze‾ ⇌ SM or H ˡ e‾ + S ⇌ SH (S = surface lattice sites)
The pseudocapacitance of ruthenium oxide (RuO 2 ) is best researched and understood . This leads to a coupled reversible redox reaction with several oxidation states, the potentials of which overlap. The electrons mostly come from the valence orbitals of the electrode material and the electron transfer reaction happens very quickly, whereby high currents can flow according to the following reaction equation:
In this charge-transfer transition (charge-transfer transition), H + protons are stored in or removed from the ruthenium crystal lattice during charging or discharging . There is a Faraday or electrochemical storage of electrical energy without chemical conversion of the electrode material. The OH groups are deposited as a molecular layer on the electrode surface. Since the measurable voltage from the redox reaction is proportional to the state of charge, the behavior of the reaction corresponds to that of a capacitor and not that of an accumulator, in which the voltage is largely independent of the state of charge.
These electron exchange reactions are very fast, much faster than the chemical processes in batteries. In these reversible reactions, one electron is transferred to the surface atoms of the negative electrode. This electron flows through the external circuit to the positive electrode. At the same time, the same number of anions migrate through the electrolyte from the negative to the positive electrode. There, in electrodes made of transition metal oxides, however, it is not the enriched anions that take up the electron again, but rather the transition metal ions that are present there and are strongly ionized in the charged state and therefore quite “electron-hungry” . Since these pseudocapacitive reactions do not produce solid chemical compounds, they can theoretically be repeated indefinitely. That is the reason for the very high cycle stability of many supercapacitors with high pseudocapacitance.
The pseudocapacitive property of a supercapacitor can be recognized with a so-called " cyclic voltammogram ", the recording of the current curve with cyclically changing voltage. The current curve of a pseudo capacitor differs significantly from that of an ideal or a lossy capacitor with purely static storage. The voltammogram of an ideal capacitor is rectangular. For a lossy capacitor, the curve shifts to a parallelogram. In electrodes with Faraday exchange reactions, the electrical charge stored in the capacitor is strongly dependent on the potential of the electrode. Because the potential of the electrode deviating from the potential of the voltammetric measurement causes a delay when driving backwards, the voltammogram of a pseudocapacitor deviates from the shape of the parallelogram, see diagram on the right.
As with double-layer electrodes , the storage capacity of pseudocapacitor electrodes results from the potential-dependent degree of coverage of the electrode surface with adsorbed ions. Since the ions are desolvated in all pseudocapacitive reactions, i. H. do not have the spherically enveloping layer of solvent molecules, they are significantly smaller than the solvated ions that contribute to the double-layer capacity. Therefore, they require correspondingly less electrode surface, which explains that with the same electrode surface, much more pseudocapacitance than double-layer capacitance can arise. This potential-dependent storage capacity also of the pseudocapacitance has the effect that, in contrast to the voltage behavior of accumulators, which have an almost charge-independent voltage curve, supercapacitors have a linear course of the capacitor voltage depending on the stored charge.
In real electrochemical capacitors, the capacitance that can be measured at the capacitor is always a combination of double-layer and pseudocapacitance. Both types of storage are inextricably linked and can only be recognized by the curve shape of the cyclic voltammogram. The proportion of pseudocapacitance of an electrode, provided it consists of a pseudocapacitively effective material such as transition metal oxides or conductive polymers, can have a value that is 10 to 100 times greater than that of the double-layer capacitance with the same electrode surface and the same volume.
Capacitors, the capacity of which comes mainly from electrochemical reactions, are called pseudocapacitors. Commercially available supercapacitors with very high pseudocapacitance combine a pseudocapacitive electrode with a double-layer electrode and belong to the family of hybrid capacitors.
literature
- Héctor D. Abruña , Yasuyuki Kiya, Jay C. Henderson: Batteries and electrochemical capacitors . In: Physics Today . No. 12 , 2008, p. 43–47 ( online [PDF]).
- F. Béguin, E. Raymundo-Piñero, E. Frackowiak: Carbons for Electrochemical Energy Storage and Conversion Systems. Chapter 8: Electrical Double-Layer Capacitors and Pseudocapacitors. CRC Press, 2009, ISBN 978-1-4200-5307-4 , pp. 329-375, doi: 10.1201 / 9781420055405-c8
- J. O'M. Bockris, MAV Devanathan, K. Muller: On the Structure of Charged Interfaces . In: Proceedings of the Royal Society . tape 274 , no. 1356 , 1963, pp. 55-79 , doi : 10.1098 / rspa.1963.0114 .
- BE Conway: Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications . Springer, Berlin 1999, ISBN 0-306-45736-9 ( limited preview in the Google book search).
- KW Leitner, M. Winter, JO Besenhard: Composite supercapacitor electrodes. In: Journal of Solid State Electrochemistry. Springer-Verlag, Volume 8, 2003, Issue 1, pp. 15-16, doi: 10.1007 / s10008-003-0412-x
- Volkmar M. Schmidt: Electrochemical process engineering. Basics, reaction technology, process optimization . Wiley-VCH, Weinheim 2003, ISBN 3-527-29958-0 , pp. 539–639 ( limited preview in Google Book Search - Chapter 7 - Electrochemical Energy Technology ).
- Yu. M. Volfkovich, TM Serdyuk: Electrochemical Capacitors. In: Russian Journal of Electrochemistry. September 2002, Volume 38, Issue 9, Kluwer Academic Publishers-Plenum Publishers, pp. 935-959, doi: 10.1023 / A: 1020220425954 .
- Jiujun Zhang, Lei Zhang, Hansan Liu, Andy Sun, Ru-Shi Liu: Electrochemical Technologies for Energy Storage and Conversion . tape 1 . Wiley-VCH, Weinheim 2011, ISBN 978-3-527-32869-7 , pp. 317–376 ( limited preview in Google Book Search - Chapter 8 - Electrochemical Supercapacitors ).
Individual evidence
- ↑ a b Zbigniew Stojek: The Electrical Double Layer and Its Structure . In: Fritz Scholz (Ed.): Electroanalytical Methods: Guide to Experiments and Applications . Springer, Berlin / Heidelberg 2010, ISBN 978-3-642-02914-1 , p. 3-10 ( online ).
- ^ A b c B. E. Conway: Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications . Springer, Berlin 1999, ISBN 0-306-45736-9 , pp. 1–8 ( limited preview in Google Book search). See also Brian E. Conway in Electrochemistry Encyclopedia: ELECTROCHEMICAL CAPACITORS Their Nature, Function, and Applications ( April 30, 2012 memento in the Internet Archive ) (accessed December 7, 2015)
- ↑ Marin S. Halper, James C. Ellenbogen: Supercapacitors: A Brief Overview. (PDF) In: MITER Nanosystems Group. March 2006, accessed May 14, 2013 . (last accessed on July 27, 2013)
- ^ A b c E. Frackowiak, F. Beguin: Carbon Materials For The Electrochemical Storage Of Energy In Capacitors. In: CARBON. 39, 2001, pp. 937-950, doi : 10.1016 / S0008-6223 (00) 00183-4 . (Review) and E. Frackowiak, K. Jurewicz, S. Delpeux, F. Béguin: Nanotubular Materials For Supercapacitors. In: Journal of Power Sources . Volumes 97-98, July 2001, pp. 822-825, doi: 10.1016 / S0378-7753 (01) 00736-4
- ↑ Josie Garthwaite: How ultracapacitors work (and why they fall short). In: Earth2Tech. GigaOM Network, July 12, 2011, accessed July 28, 2013 .
- ^ Roy Peter Richner: Development of novel bonded carbon materials for electric double-layer capacitor electrodes. DISS.ETH No. 14413, 2001, doi: 10.3929 / ethz-a-004386636
- ↑ a b B. P. Bakhmatyuk, BY Venhryn, II Grygorchak, MM Micov, SI Mudry: Intercalation Pseudo-Capacitance In Carbon Systems Of Energy Storage . In: Rev. Adv. Mater. Sci . tape 14 , 2007, p. 151-156 ( PDF ).
- ↑ a b B. E. Conway, WG Pell: Double-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid devices . In: Journal of Solid State Electrochemistry . tape 7 , no. 9 , September 2003, p. 637-644 , doi : 10.1007 / s10008-003-0395-7 .
- ↑ BE Conway, V. Birss, J. Wojtowicz: The role and utilization of pseudocapacitance for energy storage by supercapacitors . In: Journal of Power Sources . tape 66 , no. 1-2 , May 1997, pp. 1-14 , doi : 10.1016 / S0378-7753 (96) 02474-3 .
- ↑ P. Simon, Y. Gogotsi, ifc.dicp.ac.cn (PDF; 1.0 MB) Materials for electrochemical capacitors, nature materials, VOL 7, NOVEMBER 2008.
- ↑ electronics.stackexchange.com Why does an ideal capacitor give rise to a rectangular cyclic voltammogram (CV)?
- ^ AG Pandolfo, AF Hollenkamp: Carbon properties and their role in supercapacitors. In: Journal of Power Sources. Volume 157, number 1, 2006, pp. 11-27, doi : 10.1016 / j.jpowsour.2006.02.065 . (Review)
- ↑ P. Simon, A. Burke: Nanostructured carbons: Double-Layer capacitance and more. electrochem.org (PDF; 633 kB)
- ^ BE Conway: Transition from 'Supercapacitor' to 'Battery' Behavior in Electrochemical Energy Storage . In: Journal of The Electrochemical Society . tape 138 , no. 6 , May 1991, pp. 1539-1548 , doi : 10.1149 / 1.2085829 . ( PDF )
- ↑ Adam Marcus Namisnyk and JG Zhu: A Survey of Electrochemical super-capacitor technology . 2003 ( PDF [accessed December 7, 2015] Bachelor thesis; University of Technology, Sydney; 2003).
- ^ AK Shukla, S. Sampath, K. Vijayamohanan: Electrochemical supercapacitors: Energy storage beyond batteries . In: Current science . tape 79 , no. 12 , 2000, pp. 1656-1661 ( PDF ).


