Reticulomyxa filosa
| Reticulomyxa filosa | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
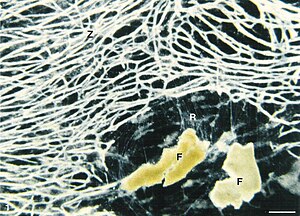
Dark field image of the cell body (Z) of Reticulomyxa filosa . At the bottom right there are feed particles (wheat germ, (F)) embedded in a fine network of reticulopodia (R). |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name of the genus | ||||||||||||
| Reticulomyxa | ||||||||||||
| Nauss, 1949 | ||||||||||||
| Scientific name of the species | ||||||||||||
| Reticulomyxa filosa | ||||||||||||
| Nauss , 1949 | ||||||||||||
Reticulomyxa filosa is a species from the group of unicellular foraminifera and the only representative of its genus from the order of the Allogromiida . This species from the protist group, first described in 1949,is one of the few foraminifera species that does not live in the sea, but in fresh water. In addition, unlike the majority of foraminifera, it also has no housing.

Since its rediscovery in the early 1980s, the species has served as a model organism for cell biological research, and since the turn of the millennium has also been used for systematic molecular genetic analyzes . Due to the work done in this context, the species has been researched unusually well for a single cell. Special features emerged in particular with regard to the unusual way of reproduction, the formation of two different stages of rest and the very efficient ways of transporting organelles within the cell.
features
The cells are almost immobile, multinucleated plasmodia, surrounded by a mucous membrane, without a housing or proteinaceous sheath with a variable, network-like shape. Their diameter is usually around 1 to 3 centimeters, they can reach a maximum size of up to 12 (according to other information up to 25) square centimeters. The organism can be divided into two areas, a central area and a peripheral area. The latter is formed by the long, thread-like reticulopodia radiating out from the central area, which is up to 6 millimeters in diameter . These are on average around 100 (40 to 250) micrometers thick along the main strands, which are often interconnected ( anastomosia ), but narrow down to 1 micrometer thick in the case of fine extensions. Overall, they can reach a length of ten times the diameter of the central area.
The cytoplasm is coarse to fine-grained and white to pale pink. During the vegetative phase it contains many non- contractile vacuoles and there is no differentiation between endo- and ectoplasm .
Ultrastructural
In the central area, the cell contains many thousands of cell nuclei with a diameter of 5 to 6 micrometers as well as dictyosomes with strong cistern stacks .
The cytoskeleton consists almost entirely of microtubules and the actin content is low. A centrosome is absent, as is a microtubule organization center in the conventional sense; the latter is instead located in the shape of a collar at the extreme end of the microtubules. The microtubules act as rails for the drive of a bidirectional granule flow , which leads away from the central area in the middle of the pseudopodia, but towards it in its periphery. The organelles move gliding on the microtubules. The build- up and breakdown of microtubules in reticulomyxa occurs at a remarkably high speed; during the build-up of the reticulopodia, stretching speeds of up to 6.5 micrometers / second were measured, and during breakdown speeds of up to 19.5 micrometers / second. It is unusual that a single microtubule is sufficient for the transport of organelles in both directions, probably because the contained motor protein dynein can work in both directions.
Resting stage and cyst
Reticulomyxa filosa has two different stages of rest, but only one of them is enveloped and therefore a cyst in the strict sense. It is not known why the species develops two different forms of resting stages. Seasonal processes or different functions are possible causes (the uncovered resting stage may represent a spreading stage).
Uncovered resting state
This dormant state, which is triggered by a lack of food, extreme excess nutrients or cold, occurs when the plasmodia contract and then fragment. The resulting cells are 50 to 100 micrometers in diameter. Apart from oversized exocytosis vesicles and missing microtubules - instead, however, numerous helical filaments aggregated to form paracrystals - the cells show no difference to active cells. A metabolism is still present.
A transition from the resting stage to the cyst form is not possible. However, just a few minutes after normal conditions prevail, the rest stages become active again and start building a new reticulopodial network.
cyst
The real cyst forms as a result of lack of food. The plasmodia contracts to a sausage-shaped shape and then breaks up into several oval to spherical plasma sections. The cell picture is almost unchanged at the beginning, only microtubules are largely missing and helical filaments are completely missing. The actual encysting then begins over the course of the next two days. Many tubular to club-shaped appendages form on the cell surface that are 20 to 40 nanometers thick and around 50 nanometers long; they are probably involved in the formation of the shell. In this phase, the shell is around 100 nanometers thick and consists of fine fibrils .
After three days, the shell is already 400 nanometers thick. At the same time, the breakdown of many organelles in the plasma has begun, and even the number of cell nuclei is reduced. The previously existing rough endoplasmic reticulum is now also missing. As a result of the degradation, dark, disorderly moving residual bodies are formed.
Encysting is over after around ten days. The cysts that emerged from the mother cell are now 50 to 200 micrometers in size, spherical or oval to bean-shaped and are often close together. They are surrounded by a 20 to 30 micrometer thick, gelatinous layer. The cyst wall is 1 to 2 micrometers thick and is reinforced from the inside by a layer of 50 nanometer thick fibrils. To the rest of the body have granules aggregated. In the approximately 500 existing 3.5 micrometer cell nuclei, the nucleoli are missing , neither microtubules nor tubulin paracrystals can be found. The cysts are now protected against dehydration and temperatures as low as −16 ° C. They remain viable for months. The cysts only reactivate when the conditions are good for several days. They use enzymes to dissolve the cyst envelope selectively, leave it and form reticulopodia again.
Way of life
Reticulomyxa filosa grows aquatic to semi-aquatic in the detritus of freshwater. The central areas are preferably in small niches or cavities, presumably covered by substrate or plants, from which only the tips of the reticulopodia protrude.
nutrition
Reticulomyxa filosa are omnivores. In addition to nutrient particles from the detritus, they also feed on smaller single and multicellular organisms such as. B. cyanobacteria , bacteria , rotifers , green algae and rarely eyelashes . The processes have not yet been documented in detail, but the corresponding particles are presumably absorbed by placing the tips of the reticulopodia over the particles or by having several of the reticulopodia flow around the respective object, unite with one another and thus enclose the food. In the case of multicellular or cell-colonial prey, individual cells are released from the organism. After this phagocytosis , the food vacuoles are transported to the central area by the granule flow. Digestion begins on the way, but is only completed in the central area, where the nutrients are then available. Accumulating excrement is excreted through the surfaces of the main strands, where they form a strong protective layer.
Reproduction
Sexual processes of Reticulomyxa filosa are not known, only an increase in cell division is documented. Cell division happens in a unique way.
During the growth of the organism, especially of the central area, new cell nuclei are continuously created. The increase in cytoplasm and cell nuclei correlate with one another. According to previous observations, the nuclear divisions within the cell are synchronized and take place within a few minutes, but not at regular intervals. In contrast to most protists, the nuclear envelope is largely retained during division and only becomes permeable to the spindle microtubules. The nucleoli attached to the nuclear envelope are also not dissolved, but given to the resulting daughter nuclei.
When the cell has exhausted the resources of its environment, it enters the last stage of reproduction, the so-called migration phase. At the beginning there is a kind of “self-cleaning” in which the cell exocytoses largely freeing the cytoplasm of residues. The previous bidirectional granule flow then becomes unidirectional and the entire cytoplasm, including that of the central area, flows outwards. After only about fifteen minutes, the cytoplasm is then distributed to the extreme ends of the reticulopodia, where three or four new central areas are usually formed. With increasing growth of the daughter cells, the plasmodia networks can then merge again at common crossing points.
Research history
Reticulomyxa filosa was discovered in a puddle full of leaves in New York City in the summer of 1937 and was first described in 1949 by the botanist Ruth N. Nauss as a slime mold due to some characteristics , although she also considered a relationship to the foraminifera. The name was suggested by Libbie H. Hyman and refers to the very long, thread-like reticulopodia. In 1982 it was found and isolated in a fish tank in the tropical houses of the Botanical Garden of the Ruhr University Bochum and shortly afterwards in a laboratory aquarium in Berkeley . Despite further finds, it was not until 1993 that it was isolated from a habitat that was not directly influenced by humans, the Möwensee near Fürstenberg / Havel .
Since, unlike almost all other foraminifera, it is easy to keep in culture and has relatively fast life cycles, since its rediscovery it has become a model organism, especially for investigations into cell motility . The same advantages - in connection with the generally rare availability of foraminifera DNA - have led to the fact that Reticulomyxa filosa has been used in many molecular genetic studies on foraminifera since the first study in 1999, occasionally also as a representative for the entire group. In 2014 the full genome of the species was published, which was unexpectedly difficult as it has a very high proportion of repetitive sequences and pseudogenes resulting from genome duplication. It is only the second member of Rhizaria that has a complete sequence.
Systematics
The systematic position of Reticulomyxa filosa was unclear until the end of the 20th century. The characteristics allowed a classification of the foraminifera as well as the slime mold. Previous systematics of the foraminifera, which are mostly based on morphological features of the shell, mostly did not take Reticulomyxa into account .
Only molecular genetic studies could clarify that the species is a foraminifera. Later investigations confirmed this and confirmed their presumed classification in the Allogromiida. Regardless of this, their exact position within the foraminifera has not yet been adequately clarified, even on the basis of molecular genetic results; indications of a basal position of Reticulomyxa in relation to all other foraminifera apart from some allogromial species are not considered established. Since it is phylogenetically in the immediate vicinity of some other caseless species as well as species with agglutinated casings, it is likely that the originally existing casing was lost in evolutionary terms as part of the adaptation to freshwater habitats.
Obviously, the species is closely related to the Wobo gigas, discovered in 1983 and first described in 2006 . It differs from Reticulomyxa filosa primarily in its proteinaceous envelope and a less complex networked central area. Wobo gigas was listed in older literature as another, hitherto undescribed reticulomyxa species.
Web links
- Video: Exercise, Ingestion, and Reproduction in Reticulomyxa Filosa . Institute for Scientific Film (IWF) 1987, made available by the Technical Information Library (TIB), doi : 10.3203 / IWF / C-1639 .
proof
- ↑ a b c Norbert Hülsmann: Biology of the genus Reticulomyxa (Rhizopoda) . In: Journal of Eukaryotic Microbiology, Vol. 31, 1984, p. 55a
- ↑ a b c d e f g h i j k Norbert Hülsmann: Exercise, food intake and reproduction in Reticulomyxa filosa (Rhizopoda) . Accompanying publication to the film C 1639, IWF Wissen und Medien gGmbH, 2006, ISSN 0073-8417
- ↑ a b c Ruth N. Nauss: Reticulomyxa filosa gen. Et spec. nov., a new primitive plasmodium . In: Bulletin of the Torrey Botanical Club, Vol. 76, 1949, pp. 161-173
- ↑ Donald D. Orokos, Richard W. Cole, Jeffrey L. Travis: Organelles Are Transported on Sliding Microtubules in Reticulomyxa . In: Cell Motility and the Cytoskeleton , 2000, Vol. 47: 4, pp. 296-306
- ↑ Manfred Schliwa, Takashi Shimizu, Ron D. Vale, Ursula Euteneuer: Nucleotide Specificities of Anterograde and Retrograde Organelle Transport in Reticulomyxa Are Indistinguishable . In: Journal of Cell Biology , 1991, vol. 112, no. 6, pp. 1199-1203
- ↑ Ralf N. Breuker: Cytoskeletal components of the plasmodial rhizopod Reticulomyxa filosa. , 1997, Dissertation to obtain the degree of Doctor of Natural Sciences in the Department of Biology at the Ruhr University Bochum, "Introduction", online
- ↑ a b c d e f Ralf N. Breuker: Cytoskeletal components of the plasmodial rhizopod Reticulomyxa filosa. , 1997, dissertation to obtain the degree of doctor of the natural sciences of the department of biology at the Ruhr University Bochum, "Cystenbildung", online
- ↑ a b Ralf N. Breuker: Cytoskeletal components of the plasmodial rhizopod Reticulomyxa filosa. 1997, Dissertation to obtain the degree of Doctor of Natural Sciences in the Department of Biology at the Ruhr University Bochum, "Cysts and stages of rest", online
- ^ Maria Holzmann, Andrea Habura, Hannah Giles, Samuel S. Bowser, Jan Pawlowski: Freshwater Foraminiferans Revealed by Analysis of Environmental DNA Samples . In: Journal of Eukaryotic Microbiology, 50 (2), 2003, pp. 135-139
- ^ Jerome Flakowski, Ignacio Bolivar, Jose Fahrni, Jan Pawlowski: Actin Phylogeny Of Foraminifera . In: Journal of Foraminiferal Research, Vol. 35, Issue 2, pp. 93-102, 2005
- ↑ John M. Archibald, David Longet, Jan Pawlowski, Patrick J. Keeling: A Novel Polyubiquitin Structure in Cercozoa and Foraminifera: Evidence for a New Eukaryotic Supergroup . In: Molecular Biology and Evolution, 20 (1): 62-66, 2003
- ↑ David Longet, John M. Archibald, Patrick J. Keeling and Jan Pawlowski: Foraminifera and Cercozoa share a common origin according to RNA polymerase II phylogenies . In: International Journal of Systematic and Evolutionary Microbiology (2003), 53, 1735-1739
- ↑ Gernot Glöckner, Norbert Hülsmann, Michael Schleicher, Angelika A. Noegel, Ludwig Eichinger, Christoph Gallinger, Jan Pawlowski, Roberto Sierra, Ursula Euteneuer, Loic Pillet, Ahmed Moustafa, Matthias Platzer, Marco Groth, Karol Szafranski, Manfred Schliwa (2014) : The Genome of the Foraminiferan Reticulomyxa filosa. Current Biology Volume 24, Issue 1: p11-18. doi : 10.1016 / j.cub.2013.11.027
- ^ Jan Pawlowski, Ignacio Bolivar, Jose F. Fahrni, Colomban De Vargas, Samuel S. Bowser: Molecular evidence that Reticulomyxa filosa is a freshwater naked foraminifer . In: Journal of Eukaryotic Microbiology, 1999, Vol. 46, pp. 612-617
- Jump up ↑ David Longet, Jan Pawlowski: Higher-level phylogeny of Foraminifera inferred from the RNA polymerase II (RPB1) gene . In: European Journal of Protistology 43 (2007) 171-177
- ↑ Jan Pawlowski, Maria Holzmann, Cédric Berney, José Fahrni, Andrew J. Gooday, Tomas Cedhagen, Andrea Habura, Samuel S. Bowser: The evolution of early Foraminifera . In: Proceedings of the National Academy of Sciences , Vol. 100, No. 20, 2003, pp. 11494-11498
- ↑ Norbert Hülsmann: Movement, food intake and reproduction in Wobo gigas gen. Et spec. nov. (Rhizopoda) , publication accompanying the film C 1638, IWF Wissen und Medien gGmbH, 2006, ISSN 0073-8417
