Sepiapterin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
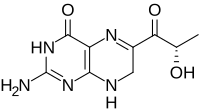
|
|||||||||||||
| General | |||||||||||||
| Surname | Sepiapterin | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 9 H 11 N 5 O 3 | ||||||||||||
| Brief description |
yellow crystal powder, drusen-shaped crystals |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 237.09 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| solubility |
soluble in water, insoluble in acetone and most organic solvents |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Sepiapterin is a chiral heterocyclic natural product from the class of pterins or pteridines .
discovery
The eyes of the fruit fly Drosophila contain yellow and red pigments, some of which are fluorescent.

A yellow pigment was discovered in Drosophila melanogaster (wild type), in increased concentration in the mutant Sepia and isolated in the 1950s by Forrest and Mitchell at the California Institute of Technology (Pasadena) in the form of yellow crystals. In addition to sepiapterin, Viscontini and Möhlmann found an isomer called isosepiapterin. The structural formula of Sepiapterin could only be determined in 1978/1979 by Max Viscontini and colleagues (Zurich) and Wolfgang Pfleiderer (Constance). It is a 7,8-dihydropterin, which is related to biopterin , but carries a C 3 chain with a carbonyl group in position 6 . The side chain has the structure of lactic acid (lactoyl residue) and has the ( S ) configuration.
Further occurrences
The compound was also found in the epidermis of the silk moth ( Bombyx mori , Mutante lem ) as well as in the skin of amphibians and fish . The traces of occurrence in the urine of mammals and humans indicate that Sepiapterin is an intermediate product (intermediate) in the metabolism of many organisms.
biochemistry
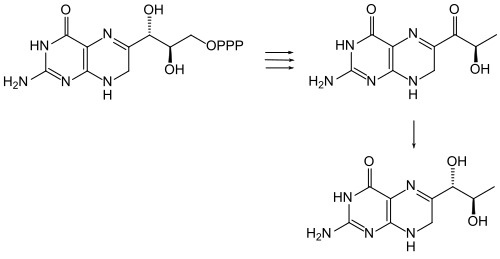
An enzymatic synthesis of sepiapterin from D -erythro-dihydroneopterin triphosphate by extracts from the kidneys of chickens has been described.
The enzyme sepiapterin reductase catalyzes the hydrogenation of the carbonyl group at C-1 'by NADPH , whereby dihydrobiopterin is formed. Finally, this is reduced to tetrahydrobiopterin (BH4).
Enzymatic formation of sepiapterin from D -erythro-dihydroneopterin triphosphate and hydrogenation to dihydrobiopterin.
Individual evidence
- ↑ a b W. Pfleiderer, Chem. Ber. , 112, pp. 2750-2755 (1979).
- ↑ a b B. Schircks, HJ Bieri, M. Viscontini, Helv. Chim. Acta , 61, pp. 2731-2738 (1978).
- ↑ a b H. S. Forrest, HK Mitchell, J. Am. Chem. Soc. , 76, p. 5656 (1954).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ E. Hadorn, KH Mitchell, Proc. Natl. Acad. Sci. USA , 37, p. 650 (1951).
- ↑ M. Viscontini, E. Möhlmann, Helv. Chim. Acta , 42, pp. 836-841 (1959).
- ↑ Wolfgang Pfleiderer: Newer developments in pteridine chemistry , Angew. Chem. , 75 , pp. 993-1014 (1963), doi : 10.1002 / anie.19630752102 .
- ↑ K. Tanaka, M. Akino, Y. Hagi, M. Doi, and T. Shiota, The enzymatic synthesis of sepiapterin by chicken kidney preparations. J. Biol. Chem. , 256, pp. 2963-2972 (1981).
- ↑ Masako Matsubara, Setsuko Katoh, Miki Akino, Seymour Kaufman: Sepiapterin reductase. In: Biochimica et Biophysica Acta - Enzymology and Biological Oxidation , 122 (2), pp. 202-212 (1966); ISSN 0926-6593 , doi : 10.1016 / 0926-6593 (66) 90062-2 .