tert -butyl carbazate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tert-butyl carbazate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 5 H 12 N 2 O 2 | |||||||||||||||
| Brief description |
white to beige crystal powder or white needles |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 132.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.1740 g cm −3 at 25 ° C |
|||||||||||||||
| Melting point |
39-42 ° C |
|||||||||||||||
| boiling point |
63-65 ° C at 0.1 hPa |
|||||||||||||||
| Vapor pressure |
1 hPa at 70 ° C |
|||||||||||||||
| solubility | ||||||||||||||||
| Refractive index |
1.4496 (25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
tert -Butyl carbazate is a hydrazine providedwith a Boc protective group and is suitable for the synthesis of substituted hydrazines, as well as other versatile tert -butyl-protected nitrogen-containing intermediates, such as. B. tert -Butylazidoformat (Boc-azide), tert- Butylhydrazodiformat (N, N'-Di-Boc-hydrazine) and its oxidation product tert -Butylazodiformat (Di- tert -Butylazodicarboxylat).
Manufacturing
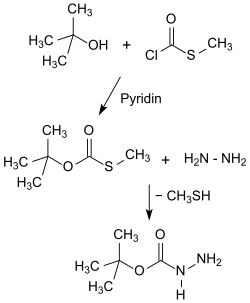
The laboratory syntheses of tert-butyl carbazate elaborated in the working group of Louis A. Carpino start in the synthesis route designated as Method I from S-methylchlorothioformate, which reacts with tert-butanol to form tert- butyl-S-methylthiol carbonate and with hydrazine hydrate in 41 to 55% strength Overall yield gives Boc-hydrazide.
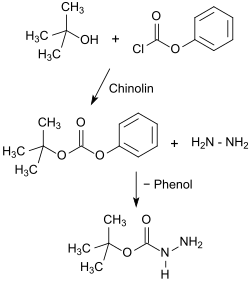
Another process variant (Method II) starts with phenyl chloroformate (phenyl chloroformate) which, with tert- butanol, gives tert- butylphenyl carbonate, which in turn reacts with hydrazine hydrate in 63 to 74% overall yield to give Boc-hydrazine.
While Method I is also suitable for larger batches and provides a crystalline product, Method II uses the poisonous and pungent-smelling phenyl chloroformate instead of the stinking thio compound, and Boc-hydrazide is obtained in significantly better yields, but as an oil that is difficult to crystallize.
Both organic synthesis methods appear to be preparatively complex, cumbersome and not very efficient.
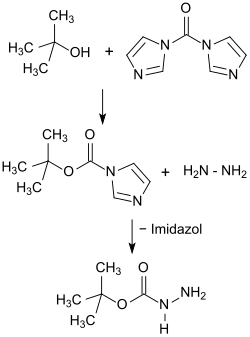
In contrast, the synthesis via Boc-imidazole from the easily accessible and easily handled carbonyldiimidazole and tert -BuOH and reaction with hydrazine appears to be much simpler and safer .
A somewhat newer alternative route starting from tert-butyl ethyl carbonate (from ethyl chloroformate and tert- butanol) and its reaction with hydrazine hydrate offers no noticeable advantages because of the long reaction times and low overall yield (28%).
properties
In its pure form, tert-butyl carbazate is a white crystalline solid that dissolves in alcohols. Because of its low melting point, the even slightly contaminated substance is in the form of an oil.
Applications
In the 1970s, tert -Butylcarbazat as a starting compound for a "carbo-tert-butoxylation agent of choice" propagated tert of processed -Butylazidoformiat to introduce Boc protecting group in peptide synthesis of many working groups. Because of its toxicity and danger (risk of explosion!), The use of tert -butyl azidoformate (Boc-azide) to introduce the Boc protective group in view of safer alternatives, such as. B. Di-tert-butyl dicarbonate (Boc anhydride, Boc 2 O) is no longer acceptable these days.
The symmetrically tert -butoxycarbonyl-substituted hydrazine tert -butylhydrazodiformate (N, N'-di-Boc-hydrazine) is obtained by tert- butoxycarbonylation of tert-butyl carbazate using Boc-azide or simpler and safer using Boc 2 O at room temperature in 85% yield accessible.
With N-bromosuccinimide di-Boc-hydrazine can be oxidized smoothly to tert -Butylazodiformiat, which as a dienophile with dienes , such as. B. Cyclopentadiene reacts in a hetero- Diels-Alder reaction to form the corresponding diazanorbornene.
A regioselective synthesis of highly substituted pyrroles with the key reagent tert -butylhydrazodiformate originates from Stephen L. Buchwald's group.
Starting from Boc-hydrazine, reaction with para-toluenesulphonic acid chloride (tosyl chloride) gives the hydrazine derivative 1-Boc-2-tosylhydrazine protected on both sides (71% yield),
with which the corresponding aldehydes are obtained in McFadyen-Stevens reactions from aliphatic and aromatic carboxylic acids under mild conditions and mostly high yields.
An important reaction step in the synthesis of the HIV protease inhibitor atazanavir is the introduction of a hydrazine group through hydrazone formation with Boc-hydrazine.
Individual evidence
- ↑ a b c d data sheet tert-Butyl carbazate, 98 +% from AlfaAesar, accessed on May 8, 2018 ( PDF )(JavaScript required) .
- ↑ a b c d data sheet tert-butyl carbazate 98% from Sigma-Aldrich , accessed on May 8, 2018 ( PDF ).
- ↑ a b L.A. Carpino, D. Collins, S. Göwecke, J. Mayo, SD Thatte, F. Tibbetts: t-Butyl carbazate in: Organic Syntheses . 44, 1964, p. 20, doi : 10.15227 / orgsyn.044.0020 ; Coll. Vol. 5, 1973, p. 166 ( PDF ).
- ↑ a b Carl. L. Yaws: The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 2nd Edition . Elsevier, Amsterdam 2015, ISBN 978-0-12-800834-8 , pp. 84 .
- ^ WH Pearson, PS Ramamoorthy, HO Sintim, J. Wang: tert -Butyl Azidoformate . In: e-EROS Encyclopedia of Reagents for Organic Synthesis . 2008, doi : 10.1002 / 047084289X.rb364.pub2 .
- ↑ a b c d L.A. Carpino, PJ Crowley: t-Butyl azodiformate In: Organic Syntheses . 44, 1964, p. 18, doi : 10.15227 / orgsyn.044.0018 ; Coll. Vol. 5, 1973, p. 160 ( PDF ).
- ↑ W. Klee, M. Brenner: tert -Butyloxycarbonyl-imidazole and tert -Butyloxycarbonyl-hydrazine . In: Helv. Chim. Acta . tape 44 , no. 7 , 1961, pp. 2151-2153 , doi : 10.1002 / hlca.19610440742 .
- ↑ M. Muraki, T. Mizoguchi: A convenient preparation of tert -Butyl carbazate . In: Chem. Pharm. Bull. Volume 18 , no. 1 , 1970, p. 217-218 , doi : 10.1248 / cpb.18.217 .
- ↑ K. Sakai, J.-P. Anselme: The direct preparation of tert -Butyl azidoformate . In: J. Org. Chem. Band 36 , no. 16 , 1971, p. 2387-2388 , doi : 10.1021 / jo00815a046 .
- ↑ M. Raju, S. Maeorg, O. Tsubrik, U. Maeorg: Efficient solventless technique for Boc-protection of hydrazines and amines . In: Arkivoc . tape VI , 2009, p. 291-297 , doi : 10.3998 / ark.5550190.0010.628 ( arkat-usa.org ).
- ↑ LA Carpino, PH Terry, PJ Crowley: Examination of synthetic routes to monosubstituted diimides. II. Synthesis of tert -butyl aryl and acylazoformates. Acid-induced cleavage of the thionocarbo- tert- butoxy group . In: J. Org. Chem. Band 26 , no. 11 , 1961, pp. 4336-4340 , doi : 10.1021 / jo01069a037 .
- ^ MR Rivero, SL Buchwald: Copper-catalyzed vinylation of hydrazides. A regioselective entry to highly substituted pyrroles . In: Org. Lett. tape 9 , no. 6 , 2007, p. 973-976 , doi : 10.1021 / ol062978s .
- ↑ Y. Iwai, T. Ozaki, R. Takita, M. Uchiyama, J. Shimokawa, T. Fukuyama: Modified McFadyen-Stevens reaction for a versatile synthesis of aliphatic / aromatic aldehydes: Design, optimization, and mechanistic investigations . In: Chem. Sci. tape 4 , no. 3 , 2013, p. 1111-1119 , doi : 10.1039 / C2SC22045H .
- ↑ L. Dalla-Vechia, B. Reichart, T. Glasnov, LSM Miranda, CO Knappe, ROMA de Souza: A three step continuous flow synthesis of the biaryl unit of the HIV protease inhibitor atazanavir . In: Org. Biomol. Chem. Band 11 , 2013, p. 6806-6813 , doi : 10.1039 / C3OB41464G .