Disulfiram
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
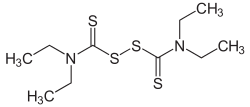
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Disulfiram | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 10 H 20 N 2 S 4 | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| Mechanism of action |
Inhibition of aldehyde dehydrogenase |
||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 296.54 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.30 g cm −3 |
||||||||||||||||||
| Melting point |
70 ° C |
||||||||||||||||||
| boiling point |
117 ° C |
||||||||||||||||||
| solubility |
Slightly soluble in water (0.2 g l −1 at 20 ° C), soluble in ethanol , acetone , diethyl ether , benzene , trichloromethane , carbon disulfide |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
DFG / Switzerland: 2 mg m −3 (measured as inhalable dust ) |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Disulfiram ( INN ), also tetraethylthiuram disulfide (TETD), ( trade name Antabus ® ) is a medicinal substance that can be used to support abstinence in the case of alcohol dependence .
alcoholism
effect
Normally, the ingested alcohol is converted to acetic acid via the intermediate stage acetaldehyde in the liver , similar to the production of vinegar through fermentation. The drug intervenes in this breakdown of the alcohol and prevents the last step in the conversion into acetic acid by blocking the enzyme aldehyde dehydrogenase . The result is that the acetaldehyde accumulates. This causes the so-called acetaldehyde syndrome , which consists in the fact that as soon as alcohol is consumed even in small doses, strong and unpleasant intolerance reactions arise, such as reddening of the skin, feeling cold in the arms and legs, nausea, headache and, above all, palpitations and drop in blood pressure up to the heart - Circulatory shock. This can even cause heart disease, angina pectoris and a heart attack in people at risk . Corresponding drugs are administered as tablets. It can also be implanted under the skin as a depot preparation .
hazards
Since the mentioned intolerance reactions can even be fatal when consuming larger amounts of alcohol ( acetaldehyde is poisonous), preparations containing disulfiram are only rarely used and in those patients who can be expected to cooperate well with the treatment. In addition, this medication may only be given after thorough preliminary investigation, e.g. B. not with severe liver damage.
See also
Various antibiotics , some antidiabetic drugs and other drugs, as well as the fungal poison coprin, have the same effect as disulfiram in combination with alcohol (see acetaldehyde syndrome ).
oncology
Recent research is investigating the effects of disulfiram as a potential cancer drug.
An epidemiological study in Danes showed that people who took disulfiram because of alcohol addiction were less likely to get breast or prostate cancer. However, the study did not show a risk reduction for melanoma.
Preclinical studies show that disulfiram inhibits the growth of tumor cells in the male prostate in vitro . In the mouse model, too, the drug reduced the tumor growth rate by up to 40%, but could not completely inhibit it. In vitro studies on breast cancer cells showed that disulfiram induces apoptosis in tumor cells, but not in healthy cells of the breast . Disulfiram has also been found to act on tumor cells in non-small cell lung cancer and in glioblastoma .
The exact mechanism of this effect on cancer cells is not known and is still being investigated. The formation of complexes of the active metabolite, dithiocarb, which is formed in the liver from disulfiram, with copper may play a role. Disulfiram is more effective in inhibiting the growth of tumor cells when given in combination with copper.
As of today (December 2017) there is only one clinical study on 40 patients with non-small cell lung cancer in which one group received disulfiram in addition to chemotherapy and was compared with a group that received only chemotherapy. In the group with disulfiram, the mean survival time was increased from 7.1 to 10 months. However, the informative value of this study is limited by the small number of cases; further clinical studies are being carried out.
Other uses
Disulfiram is used as an aid in the vulcanization of rubber. It is historically interesting that the alcohol-aversive effect was allegedly first noticed by chance in workers in this area.
Individual evidence
- ↑ a b c d e f g h Entry on tetraethylthiuram disulfide. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ a b Entry on disulfiram in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ Entry on Disulfiram in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 97-77-8 or disulfiram ), accessed on November 2, 2015.
- ↑ Entry on disulfiram in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Specialist information for antabuse in the Swiss drug compendium .
- ↑ Emminger, H. Thomas, K. EXAPLAN Volume 2; 6th ed .; P. 2141.
- ↑ Boris Cvek in: Drug Repurposing for Terminal-Stage Cancer Patients . American Journal of Public Health, Vol. 106, No. 6, June 2016, pp. E3-e3.
- ↑ Diethyldithiocarbamate complexes with metals used as food supplements show different effects in cancer cells , with Jindřich Sedláček (Palacky University, Olomouc), Luisa MDRS Martins (ISEL Lisbon / Technical University Lisbon, Portugal) Petr Daněk (Palacky University, Olomouc), Armando JL Pombeiro (Technical University Lisbon, Portugal), Journal of Applied Biomedicine, Vol. 12, No. 4, November 2014, pp. 301-308.
- ↑ Nonprofit drugs as the salvation of the world's healthcare systems: the case of Antabuse (disulfiram) . Drug Discovery Today 2012.
-
↑ Antabuse repurposing: we need more knowledge and wide international support . International Journal of Cancer 2011
The Ubiquitin-Proteasome System (UPS) and the Mechanism of Action of Bortezomib , with Dvořák Z., Current Pharmaceutical Design 2011. - ↑ Targeting Malignancies with Disulfiram (Antabuse): Multidrug Resistance, Angiogenesis, and Proteasome . Current Cancer Drug Targets (IF = 4.327) 2011.
- ↑ Antabuse (disulfiram) as a pilot case of nonprofit drug . International Journal of Cancer 2010.
- ↑ Gro Askgaard, Søren Friis, Jesper Hallas, Lau C. Thygesen, Anton Pottegård: Use of disulfiram and risk of cancer . In: European Journal of Cancer Prevention . tape 23 , no. 3 , p. 225–232 , doi : 10.1097 / cej.0b013e3283647466 ( wkhealth.com [accessed December 23, 2017]).
- ↑ Kristiina Iljin, Kirsi Ketola, Paula Vainio, Pasi Halonen, Pekka Kohonen: High-Throughput Cell-Based Screening of 4910 Known Drugs and Drug-like Small Molecules Identifies Disulfiram as an Inhibitor of Prostate Cancer Cell Growth . In: Clinical Cancer Research . tape 15 , no. 19 , October 1, 2009, p. 6070-6078 , doi : 10.1158 / 1078-0432.ccr-09-1035 .
- ↑ Di Chen, Qiuzhi Cindy Cui, Huanjie Yang, Q. Ping Dou: Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity . In: Cancer Research . tape 66 , no. 21 , November 1, 2006, pp. 10425-10433 , doi : 10.1158 / 0008-5472.CAN-06-2126 , PMID 17079463 .
- ↑ Lincan Duan, Hongmei Shen, Guangqiang Zhao, Runxiang Yang, Xinyi Cai: Inhibitory effect of Disulfiram / copper complex on non-small cell lung cancer cells . In: Biochemical and Biophysical Research Communications . tape 446 , no. 4 , p. 1010-1016 , doi : 10.1016 / j.bbrc.2014.03.047 .
- ↑ Joanna Triscott, Mary Rose Pambid, Sandra E. Dunn: Concise Review: Bullseye: Targeting Cancer Stem Cells to Improve the Treatment of Gliomas by Repurposing Disulfiram . In: STEM CELLS . tape 33 , no. 4 , April 1, 2015, p. 1042-1046 , doi : 10.1002 / stem.1956 .
- ↑ Zdenek Skrott, Martin Mistrik, Klaus Kaae Andersen, Søren Friis, Dusana Majera: Alcohol-abuse drug disulfiram targets cancer via p97 segregase adapter NPL4 . In: Nature . tape 552 , no. 7684 , December 2017, doi : 10.1038 / nature25016 .
- ↑ Gro Askgaard, Søren Friis, Jesper Hallas, Lau C. Thygesen, Anton Pottegård: Use of disulfiram and risk of cancer . In: European Journal of Cancer Prevention . tape 23 , no. 3 , p. 225–232 , doi : 10.1097 / cej.0b013e3283647466 ( wkhealth.com [accessed December 23, 2017]).