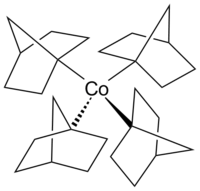
Tetrakis (1-norbornyl) cobalt (IV)
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Tetrakis (1-norbornyl) cobalt (IV) | ||||||
| other names |
( T -4) -Tetrakis (bicyclo [2.2.1] hept-1-yl) cobalt |
||||||
| Molecular formula | T4 - [Co (nor) 4 ], C 28 H 44 Co | ||||||
| Brief description |
brown crystals |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 439.58 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| Melting point |
100 ° C (decomposition) |
||||||
| solubility |
soluble in THF |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Tetrakis (1-norbornyl) cobalt (IV) is an air-sensitive, organometallic compound of cobalt . It was first presented by Barton K. Bower and Howard G. Tennent in 1972 and is one of the rare compounds in which cobalt is assigned the formal oxidation state + IV.
presentation
Tetrakis (1-norbornyl) cobalt (IV) can be obtained by the disproportionation reaction of CoCl 2 • THF with 1-norbornyllithium (norLi) in n- pentane under protective gas . The cobalt (II) chloride-THF adduct is by Soxhlet extraction of anhydrous CoCl 2 with THF and the organolithium compound by reaction of 1-chloro norbornane with metallic lithium accessible.
The purification takes place via filtration and recrystallization .
properties
In this cobalt complex is the first thermally stable homoleptic cobalt (IV) tetra organyl , with only σ -binding ligands . It is also the first isolated low-spin complex with a tetrahedral structure.
stability
The special stability is largely due to the fact that neither α - another β -hydride elimination to take place. In the α- position to the metal (corresponds to the 1-position of the norbornyl ligand) no further hydrogen is bonded and a hydride elimination in the β- position would lead to an energetically unfavorable double bond at the bridgehead atom ( Bredt's rule ). In addition, the bulky norbornyl ligands cause steric shielding of the central atom and prevent rapid ligand substitution and homolysis , with dimerization of the ligands.
The rare d 5 low-spin configuration in the tetrahedral ligand field is achieved in that the ligand, as a very strong σ - or more precisely n - electron pair donor , increases the splitting of the e and t 2 orbital sets in such a way that the spin pairing energy can be overcome. The result is an e 4 t 2 1 configuration and consequently a magnet measurement shows paramagnetism in the order of magnitude of an unpaired electron.
Cobalt (III) and Cobalt (V) analogues
Starting from CoCl 2 • THF and 1-norbornyllithium (norLi), a trivalent cobalt complex can be prepared. If a mixture of diethyl ether and THF is used as a solvent instead of n- pentane , disproportionation occurs with the formation of a tetrakis (1-norbornyl) cobaltate (III) complex, which crystallizes with solvated lithium counterion and elemental cobalt .
The compound is sensitive to air, is green in color, and is paramagnetic, to the extent of two unpaired electrons. As a result, there is also a low-spin configuration with a tetrahedral structure (d 6 , e 4 t 2 2 ).
If tetrakis (1-norbornyl) cobalt (IV) is oxidized with Ag [BF 4 ] in THF , a pentavalent cobalt complex is obtained, which crystallizes with a tetrafluoroborate anion as a counterion.
This complex is the first to be isolated in which cobalt is in the + V oxidation state. This is also a low-spin complex (d 4 , e 4 t 2 0 ).
Individual evidence
- ↑ a b c d e f B. K. Bower and HG Tennent: Transition metal bicyclo [2.2.1] hept-1-yls . In: J. Am. Chem. Soc. . 94, No. 7, 1972, pp. 2512-2514. doi : 10.1021 / ja00762a056 .
- ↑ a b c d e f g E. K. Byrne, KH Theopold: Synthesis, characterization, and electron-transfer reactivity of norbornyl complexes of cobalt in unusually high oxidation states . In: J. Am. Chem. Soc. . 111, No. 11, 1989, pp. 3887-3896. doi : 10.1021 / ja00193a021 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1695.
- ↑ a b E. K. Byrne, DS Richeson and KH Theopold: Tetrakis (1-norbornyl) cobalt, a low spin tetrahedral complex of a first row transition metal . In: J. Chem. Soc., Chem. Commun. . No. 19, 1986, pp. 1491-1492. doi : 10.1039 / C39860001491 .
- ↑ C. Janiak, T. Klapötke , H.-J. Meyer, R. Alsfasser, E. Riedel (Ed.): Moderne Anorganische Chemie , 3rd edition, De Gruyter, 2007, ISBN 978-3-11-020685-2 , p. 718.
- ↑ a b c d E. K. Byrne, KH Theopold: Redox chemistry of tetrakis (1-norbornyl) cobalt. Synthesis and characterization of a cobalt (V) alkyl and self-exchange rate of a Co (III) / Co (IV) couple . In: J. Am. Chem. Soc. . 109, No. 4, 1987, pp. 1282-1283. doi : 10.1021 / ja00238a066 .
![{\ displaystyle \ mathrm {CoCl_ {2} \ cdot THF \ + \ 4 \ norLi \ {\ xrightarrow [{}] {Pentane}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5f72189a0ab34a5cb4190d64ebe502de0412c1c7)
![{\ displaystyle \ mathrm {\ 1/2 \ [Co (nor) _ {4}] \ + \ 1/2 \ Co \ + \ 2 \ LiCl \ +}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/bffdd75a8662177b111cf6b528965bfab4384580)


![{\ displaystyle \ mathrm {3 \ CoCl_ {2} \ cdot THF \ + \ 8 \ norLi \ + \ 5 \ THF \ {\ xrightarrow [{}] {Et_ {2} O / THF}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cab23573b8be7ac95264970ffff9d59e928ba0aa)
![{\ displaystyle \ mathrm {\ 2 \ [Li (THF) _ {4}] ^ {+} [Co (nor) _ {4}] ^ {-} \ + \ Co \ +}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/714d570c5f847df646024eef5838dfee953fce27)

![{\ displaystyle \ mathrm {[Co (nor) _ {4}] \ + \ Ag [BF_ {4}] \ {\ xrightarrow [{}] {THF}} \ [Co (nor) _ {4}] ^ {+} [BF_ {4}] ^ {-} \ + \ Ag}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/beb2aad3f5219403286f4d4c0a07422ed670e6b2)