Titanium tetraethanolate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
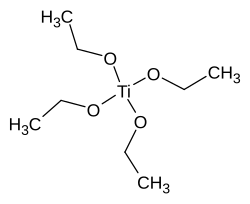
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Titanium tetraethanolate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 8 H 20 O 4 Ti | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 228.15 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| boiling point |
150–155 ° C (at 16 h Pa ) |
||||||||||||||||||
| solubility |
hydrolyzed in water |
||||||||||||||||||
| Refractive index |
1.505 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Titanium tetraethanolate is a chemical compound from the group of alcoholates or titanium organic compounds.
properties
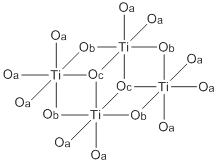
Titanium tetraethanolate is a white, crystalline solid that melts at 38–40 ° C. The compound also occurs as a colorless to yellowish liquid with an alcoholic odor, this being due to a metastable, supercooled melt or contamination by hydrolysis products. The solid forms a monoclinic crystal lattice with the space group C2 / c . This has a tetrameric structure, with the titanium atoms coordinated in an octahedral manner. There are three different binding variants for the ethanolate function with single, double and triple coordinated oxygen atoms. Different Ti-O bond lengths result from the different coordination. Trimeric structures occur in the liquid phase and in solution.
The alcoholate hydrolyzes in water and is soluble in toluene and acetone .
It has a viscosity of 55 to 65 mPa · s at 20 ° C. Technical titanium tetraethanolate contains 5 to 15% isopropanol .
use
Titanium tetraethanolate can be used to produce titanium dioxide layers and nanoparticles.
safety instructions
The vapors of titanium tetraethanolate can form an explosive mixture with air ( flash point 33 ° C).
Individual evidence
- ↑ a b c Crowe, RW; Caughlan, CN: The Electric Moments of Tetraethoxytitanium, Monochlorotriethoxytitanium and Trichlorophenoxytitanium in Benzene Solutions. I in J. Am. Chem. Soc. 72 (1950) 1694-1697, doi : 10.1021 / ja01160a076 .
- ↑ a b c d e Bradley, DC; Gaze, R .; Wardlaw, W .: The hydrolysis of titanium tetraethoxide in J. Chem. Soc. 1955, 721-726, doi : 10.1039 / JR9550000721 .
- ↑ a b Bradley, DC; Prevedorou, CCA; Swanwick, JD; Wardlaw, W .: Parachors and molecular structure of some metal alkoxides in J. Chem. Soc. 1958, 1010-1014, doi : 10.1039 / JR9580001010 .
- ↑ a b Caughlan, CN Smith, HS; Katz, W .; Hodgson, W .; Crowe, RW: Organic Compounds of Titanium. II. Association of Organic Titanates in Benzene Solution in J. Am. Chem. Soc. 73 (1951) 5652-5654, doi : 10.1021 / ja01156a046 .
- ↑ a b c Ibers, JA: Crystal and Molecular Structure of Titanium (IV) Ethoxide in Nature 197 (1963) 686-687, doi : 10.1038 / 197686a0 .
- ↑ a b Turevskaya, EP: Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya 1977, (7), 1463-1466.
- ↑ a b c Entry on titanium tetraethanolate in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b Data sheet titanium tetraethanolate (PDF) from Merck , accessed on February 20, 2010.
- ↑ Data sheet Titanium (IV) ethoxide from Sigma-Aldrich , accessed on April 24, 2011 ( PDF ).
- ↑ Martin, RL; Winter, G .: Association of Titanium (IV) Alkoxides in Benzene in Nature 197 (1963) 687, doi : 10.1038 / 197687a0 .
- ↑ Data sheet titanium tetraethanolate from Acros, accessed on February 20, 2010.
- ↑ Nurul Huda Yusoff, Muhamad Mat Salleh, Muhammad Yahaya: Fluorescence gas sensor using TiO2 nanoparticles coated with porphyrin dye thin films . In: Solid State Science and Technology, Vol. 16, No. 1 (2008) pp. 63-74.