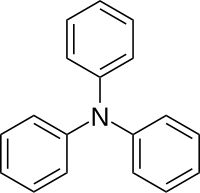
Triphenylamine
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Triphenylamine | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 18 H 15 N | ||||||||||||||||||
| Brief description |
colorless to white crystals with a characteristic odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 245.33 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.774 g cm −3 |
||||||||||||||||||
| Melting point |
125-127 ° C |
||||||||||||||||||
| boiling point |
347-348 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Triphenylamine is an aromatic organic compound with the molecular formula C 18 H 15 N. It belongs to the class of tertiary amines .
Extraction and presentation
Triphenylamine can be obtained from diphenylamine by the action of sodium and bromobenzene .
properties
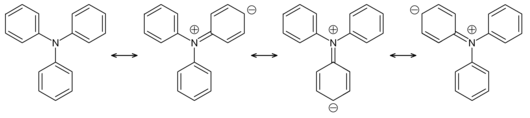
In contrast to most other aliphatic and aromatic amines , triphenylamine does not have a strongly basic character with a pK B value of 19. This is due to the resonance structures of the molecule, which significantly weaken the nucleophilicity of the central nitrogen atom.
Triphenylamine and its derivatives have useful properties in their electrical conductivity and electroluminescence . For this reason, they are used as hole transporters in OLED materials.
gallery
3D structure of triphenylamine in a ball and stick model
3D structure of triphenylamine in the space-filling model
Individual evidence
- ↑ a b c d e f g h Entry on triphenylamine. In: Römpp Online . Georg Thieme Verlag, accessed on September 4, 2019.
- ↑ a b Entry on triphenylamine in the GESTIS substance database of the IFA , accessed on September 5, 2019(JavaScript required) .
- ↑ a b data sheet triphenylamine, 98% from Sigma-Aldrich , accessed on September 5, 2019 ( PDF ).
- ↑ Touraj Manifar, Sohrab Rohani: Synthesis and Analysis of Triphenylamine: A Review. In: The Canadian Journal of Chemical Engineering . 82, 2004, pp. 323-334, doi : 10.1002 / cjce.5450820213 .
- ↑ periodic table online , accessed on September 4, 2019.
- ↑ Wei Shi, Suqin Fan, Fei Huang, Wei Yang, Ransheng Liu, Yong Cao: Synthesis of Novel triphenylamine-based Conjugated Polyelectrolytes and Their Application to hole-transport layer in Polymeric Light-Emitting Diodes . In: J. Mater. Chem. , 2006, 16, 2387-2394. doi : 10.1039 / B603704F