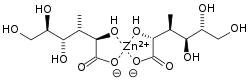
Zinc gluconate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Zinc gluconate | |||||||||||||||
| Molecular formula | C 12 H 22 O 14 Zn | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| Drug information | ||||||||||||||||
| ATC code | ||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 455.67 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
155 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Zinc gluconate is a chemical compound and is the zinc salt of gluconic acid .
Extraction and presentation
Zinc gluconate can be obtained by reacting gluconic acid with zinc oxide and zinc acetate .
properties
Zinc gluconate is a white, odorless solid that is soluble in water. It occurs in several hydrate forms up to the trihydrate.
use
As a zinc compound, zinc gluconate is used to prevent or treat zinc deficiency conditions. The WHO recommends zinc supplementation as a complementary measure in the treatment of dehydration caused by diarrhea with oral rehydration solution (ORS) . In terms of health, zinc compounds such as zinc gluconate are also used in the prevention or treatment of colds. For zinc in food supplements, the European Food Safety Authority (EFSA) has approved the health-related claim "contributes to the normal function of the immune system (physical defense)".
Two placebo- controlled double-blind studies have shown that taking zinc gluconate lozenges reduces the severity of cold symptoms by around 40 percent and shortens their duration by three to four days , although this can be associated with various side effects. In the Sherif B. Mossad study, zinc gluconate lozenges with 13.3 mg zinc content were taken every two hours while awake, which corresponds to a daily intake of max. 7–8 lozenges were equivalent. The recommended tolerable upper intake level of the European Food Safety Authority is 25 mg zinc per day; Due to possible immunodeficiency, the study avoided zinc doses greater than 150 mg per day.
In an evaluation ( meta-analysis ) carried out for the Cochrane Collaboration of a total of 15 published clinical studies on the treatment and prevention of colds with zinc, the Indian Institute of Medical Education and Research was able to determine a mitigating effect and shortening the duration of the illness. According to a study published in 1998, zinc gluconate in the form of lozenges had no significant positive effects on the duration of a cold in children and adolescents. In adults, five out of ten studies reported the positive effects of zinc administration for colds, a faster resolution of the symptoms of the disease. In contrast, no effectiveness was found in the other five studies. In a controlled study, zinc gluconate administered intranasally had no effect on the severity or duration of cold symptoms associated with rhinovirus infections . A permanent loss of the sense of smell ( anosmia ) has been reported in isolated cases following intranasal administration of zinc gluconate .
Individual evidence
- ↑ a b c d e f g h data sheet zinc gluconate (PDF) from Merck , accessed on February 14, 2016.
- ^ GWA Milne: Gardner's Commercially Important Chemicals Synonyms, Trade Names, and Properties . John Wiley & Sons, 2005, ISBN 0-471-73661-9 , pp. 739 ( limited preview in Google Book search).
- ↑ Hui Feng, Zhiwei Xu, Qianfeng Li, Xiuru Wang, Qiuyue Song: One pot synthesis, characterization and mechanism of zinc glycinate monohydrate . May 30, 2015, doi : 10.2991 / asei-15.2015.420 .
- ↑ George A. Burdock: Encyclopedia of Food and Color Additives . CRC Press, 1997, ISBN 978-0-8493-9414-0 , pp. 2999 ( limited preview in Google Book search).
- ^ WU Khan, DW Sellen: Zinc supplementation in the management of diarrhea , WHO April 2011
- ↑ European Food Safety Authority: Scientific Opinion: Chromium picolinate, zinc picolinate and zinc picolinate dihydrate added to food supplements for nutritional purposes. from 2009, full text ( Memento of the original from August 10, 2013 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF).
- ^ Sherif B. Mossad: Zinc Gluconate Lozenges for Treating the Common Cold. In: Annals of Internal Medicine. No. 125, 1996, p. 81, doi: 10.7326 / 0003-4819-125-2-199607150-00001 .
- ↑ JC Godfrey, B. Conant Sloane, DS Smith, JH Turco, N. Mercer, NJ Godfrey: Zinc gluconate and the common cold: a controlled clinical study. In: The Journal of international medical research. Volume 20, Number 3, June 1992, pp. 234-246, ISSN 0300-0605 , PMID 1397668 .
- ↑ Meenu Singh, Rashmi R. Das: Zinc for the common cold (Intervention Review) . The Cochrane Library . Retrieved February 17, 2011.
- ↑ Zinc helps with colds . Spiegel Online , February 16, 2011.
- ↑ Zinc gluconate does not work in children , deutsche-apotheker-zeitung.de, accessed on February 14, 2016.
- ↑ Zinc Therapy German Green Cross for Health e. V., accessed on February 16, 2016.
