Tin (II) oxalate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
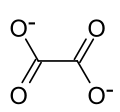
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Tin (II) oxalate | |||||||||||||||
| Molecular formula | SnC 2 O 4 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 206.71 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.55 g cm −3 |
|||||||||||||||
| Melting point |
280 ° C (decomposition) |
|||||||||||||||
| solubility |
poor in water (0.5 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Tin (II) oxalate is a chemical compound of tin from the group of oxalates (i.e. a salt of oxalic acid ) that is used, among other things, as a catalyst .
properties
When tin (II) oxalate is heated, the compound decomposes to tin (II) oxide .
use
Tin (II) oxalate is used as
- Catalyst for the production of esters
- Catalyst in the hydrogenation of coal (up to 0.06%)
- Reducing agents in glass production
- in the textile industry
Individual evidence
- ↑ a b c d e data sheet tin (II) oxalate (PDF) from Merck , accessed on April 26, 2011.
- ↑ a b Entry on tin (II) oxalate in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ^ Heinz-Gerhard Franck, Jürgen Walter Stadelhofer: Industrial Aromatic Chemistry: Raw Materials · Processes · Products . Springer, 1987, ISBN 978-3-662-07876-1 , pp. 51 .
- ↑ MOMCPL: Product Range

