Chemistry: Difference between revisions
→Chemical energetics: moved a sentence around to improve the flow |
→History: added some wikilinks |
||
| Line 35: | Line 35: | ||
{{see also|Alchemy|Timeline of chemistry|Nobel Prize in Chemistry}} |
{{see also|Alchemy|Timeline of chemistry|Nobel Prize in Chemistry}} |
||
The genesis of chemistry can be traced to the phenomenon of [[combustion|burning]] which led to [[metallurgy]]- the art and science of processing ores to get metals. Later on the science formation of [[alloys]] was discovered as a method for strengthening metals. Thus, Gold had been purified long before the first alloys were created, even though, the underlying process for purifying gold was not well understood. It was thought to be a transformation rather than purification. Many scholars in those days thought it reasonable to find a means for transforming cheaper (base) metals into gold. This led to the rise of alchemy, and the search for the Philosopher's Stone, believed to be a potent mean to bring about such a transformation. This development took place over thousands of years.<ref>Chemical Heritage Foundation: Ancients and Alchemists [http://www.chemheritage.org/explore/ancients-time.html]</ref> |
The genesis of chemistry can be traced to the phenomenon of [[combustion|burning]] which led to [[metallurgy]]- the art and science of processing ores to get metals. Later on the science formation of [[alloys]] was discovered as a method for strengthening metals. Thus, Gold had been purified long before the first alloys were created, even though, the underlying process for purifying gold was not well understood. It was thought to be a transformation rather than purification. Many scholars in those days thought it reasonable to find a means for transforming cheaper (base) metals into gold. This led to the rise of alchemy, and the search for the [[Philosopher's Stone]], believed to be a potent mean to bring about such a transformation. This development took place over thousands of years.<ref>Chemical Heritage Foundation: Ancients and Alchemists [http://www.chemheritage.org/explore/ancients-time.html]</ref> |
||
It is often said that medieval [[Muslim]] were the earliest chemists, this claim is particularly based on the works of [[Geber]] (d. 815), [[Al-Kindi]] (d. 873), [[Al-Razi]] (d. 925), and [[Abu-Rayhan Biruni]] (d. 1048).<ref>[[Will Durant]] (1980), ''The Age of Faith (The Story of Civilization, Volume 4)'', p. 162-186, Simon & Schuster, ISBN 0671012002: |
It is often said that medieval [[Muslim]] were the earliest chemists, this claim is particularly based on the works of [[Geber]] (d. 815), [[Al-Kindi]] (d. 873), [[Al-Razi]] (d. 925), and [[Abu-Rayhan Biruni]] (d. 1048).<ref>[[Will Durant]] (1980), ''The Age of Faith (The Story of Civilization, Volume 4)'', p. 162-186, Simon & Schuster, ISBN 0671012002: |
||
<br>{{quote|"Chemistry as a science was almost created by the Muslims; for in this field, where the Greeks (so far as we know) were confined to industrial experience and vague [[hypothesis]], the [[Saracen]]s introduced precise [[observation]], controlled [[experiment]], and careful records. They invented and named the [[alembic]] (al-anbiq), chemically analyzed innumerable [[substance]]s, composed [[Lapidary|lapidaries]], distinguished [[alkali]]s and [[acid]]s, investigated their affinities, studied and manufactured hundreds of [[drug]]s. Alchemy, which the Muslims inherited from Egypt, contributed to chemistry by a thousand incidental discoveries, and by its method, which was the most scientific of all medieval operations."}}</ref><ref>Dr. K. Ajram (1992), ''Miracle of Islamic Science'', Appendix B, Knowledge House Publishers, ISBN 0911119434:<br>{{quote|"[[Alexander von Humboldt|Humboldt]] regards the Muslims as the founders of chemistry."}}</ref> The works of Geber became more widely known in Europe through [[Latin]] translations by a [[pseudo-Geber]] in 14th century [[Spain]], who also wrote some of his own books under the pen name "Geber". |
<br>{{quote|"Chemistry as a science was almost created by the Muslims; for in this field, where the Greeks (so far as we know) were confined to industrial experience and vague [[hypothesis]], the [[Saracen]]s introduced precise [[observation]], controlled [[experiment]], and careful records. They invented and named the [[alembic]] (al-anbiq), chemically analyzed innumerable [[substance]]s, composed [[Lapidary|lapidaries]], distinguished [[alkali]]s and [[acid]]s, investigated their affinities, studied and manufactured hundreds of [[drug]]s. Alchemy, which the Muslims inherited from Egypt, contributed to chemistry by a thousand incidental discoveries, and by its method, which was the most scientific of all medieval operations."}}</ref><ref>Dr. K. Ajram (1992), ''Miracle of Islamic Science'', Appendix B, Knowledge House Publishers, ISBN 0911119434:<br>{{quote|"[[Alexander von Humboldt|Humboldt]] regards the Muslims as the founders of chemistry."}}</ref> The works of Geber became more widely known in Europe through [[Latin]] translations by a [[pseudo-Geber]] in 14th century [[Spain]], who also wrote some of his own books under the pen name "Geber". |
||
One of the main factors that led to the emergence of chemistry in Europe was the recurrent incidence of the [[plague]] and blights in Europe during the so called [[Dark Ages]]. This gave rise to a need for medicines. It was thought that there might exist a universal medicine that can cure all diseases - called the Elixir of Life. However, like the Philosopher's Stone, neither one were ever found. Modern day chemistry states that such a medicine is not possible. Alchemy for many was an avenue for charlatans to create fake medicines. |
One of the main factors that led to the emergence of chemistry in Europe was the recurrent incidence of the [[plague]] and blights in Europe during the so called [[Dark Ages]]. This gave rise to a need for medicines. It was thought that there might exist a universal medicine that can cure all diseases - called the [[Elixir of Life]]. However, like the Philosopher's Stone, neither one were ever found. Modern day chemistry states that such a medicine is not possible. Alchemy for many was an avenue for charlatans to create fake medicines. |
||
For many practitioners of the so called alchemy, it was an intellectual pursuit that could not separate superstition from scientific inquiry. Over time, practitioners got better at it. [[Paracelsus]] (1493-1541) rejected the 4-elemental theory and with only a vague understanding of his chemicals and medicines, formed a hybrid of alchemy and science in what was to be called ''[[iatrochemistry]]''. The influences of philosophers such as [[Sir Francis Bacon]] (1561-1626) and [[René Descartes]] (1596-1650), led to a scientific revolution. These philosophers demanded more rigor in mathematics and in removing bias from scientific observations. In chemistry, this began with [[Robert Boyle]] (1627-1691), who discovered gases, and came up with equations that were known as [[Boyle's Law]].<ref>BBC - History - Robert Boyle (1627 - 1691) [http://www.bbc.co.uk/history/historic_figures/boyle_robert.shtml]</ref> The person often termed as the Father of Chemistry is [[Antoine Lavoisier]] (1743-1794), who developed the theory of [[Conservation of mass]] in 1783. Equally important was the development of the Atomic Theory, principally by [[John Dalton]] (1766-1844) around 1800. |
For many practitioners of the so called alchemy, it was an intellectual pursuit that could not separate superstition from scientific inquiry. Over time, practitioners got better at it. [[Paracelsus]] (1493-1541) rejected the 4-elemental theory and with only a vague understanding of his chemicals and medicines, formed a hybrid of alchemy and science in what was to be called ''[[iatrochemistry]]''. The influences of philosophers such as [[Sir Francis Bacon]] (1561-1626) and [[René Descartes]] (1596-1650), led to a scientific revolution. These philosophers demanded more rigor in mathematics and in removing bias from scientific observations. In chemistry, this began with [[Robert Boyle]] (1627-1691), who discovered gases, and came up with equations that were known as [[Boyle's Law]].<ref>BBC - History - Robert Boyle (1627 - 1691) [http://www.bbc.co.uk/history/historic_figures/boyle_robert.shtml]</ref> The person often termed as the Father of Chemistry is [[Antoine Lavoisier]] (1743-1794), who developed the theory of [[Conservation of mass]] in 1783. Equally important was the development of the Atomic Theory, principally by [[John Dalton]] (1766-1844) around 1800. |
||
Revision as of 08:20, 26 June 2007
Editing of this article by new or unregistered users is currently disabled due to vandalism. See the protection policy and protection log for more details. If you cannot edit this article and you wish to make a change, you can submit an edit request, discuss changes on the talk page, request unprotection, log in, or create an account. |
Chemistry (from Egyptian kēme (chem), meaning "earth"[1]) is the science treating matter at the atomic to macromolecular scale, the reactions, transformations and aggregations of matter, as well as accompanying energy and entropy changes during these processes. In short, chemistry studies molecules, crystals, and metal/nonmetals and is concerned with the composition and statistical properties of such structures, as well as their transformations and interactions to become materials encountered in everyday life. According to quantum mechanics, all physical and chemical properties of materials are generally determined by their structure at the molecular or atomic scale, which is itself defined by interatomic electromagnetic forces, and laws of quantum mechanics. Robert Boyle (1661), Antoine Lavoisier (1787), and John Dalton (1808) can be considered the three fathers of modern chemistry,[2] while some consider the earlier chemist Geber (d. 815) to be the "father of chemistry".[3]
Introduction
Chemistry is the direct result of interaction of electric charges constituting atomic matter (namely electrons of atomic shells and protons of atomic nuclei) with each other according to rules of quantum mechanics. A configuration of all charges involved with minimal possible overall potential energy is what we call "a molecule" (and if there is no local energy minimum, then there is no stable molecule).
Chemistry is often called "the central science" because it connects the other natural sciences, such as astronomy, physics, material science, biology, and geology.[4][5] These connections are formed through various sub-disciplines that utilize concepts from multiple scientific disciplines. For example, physical chemistry involves applying the principles of physics to materials at the atomic and molecular level. The precise nature of the theoretical connection that chemistry (along with the other so-called special sciences) has with physics is a matter of research in philosophy of science.
Chemistry pertains to the interactions of matter. These interactions may be between two material substances or between matter and energy, especially in conjunction with the first law of thermodynamics. Traditional chemistry involves interactions between substances in chemical reactions, where one or more substances become one or more other substances.[6] Sometimes these reactions are driven by energetic (enthalpic) considerations, such as when two highly energetic substances such as elemental hydrogen and oxygen react to form the less energetic substance water. Chemists often use reaction equations to summarize a specific reaction. The chemical reaction between hydrogen and oxygen is shown in the following equation:
- 2 H2 + O2 → 2 H2O.[7]
The number of atoms on the left and the right of the arrow is always equal in chemical reactions. Other reactions are driven primarily by entropy, which, simply stated, is a measure of disorder. Chemical reactions may be facilitated by a catalyst, which is generally another chemical substance present within the reaction media but unconsumed (such as sulfuric acid catalyzing the electrolysis of water) or a non-material phenomenon (such as electromagnetic radiation in photochemical reactions). Traditional chemistry also deals with the analysis of chemicals both in and apart from a reaction, as in spectroscopy.[8]

All ordinary matter consists of atoms or the subatomic components that make up atoms; protons, electrons and neutrons.[9] Atoms may be combined to produce more complex forms of matter such as ions, molecules or crystals. The structure of the world we commonly experience and the properties of the matter we commonly interact with are determined by properties of chemical substances and their interactions. Steel is harder than iron because its atoms are bound together in a more rigid crystalline lattice. Wood burns or undergoes rapid oxidation because it can react spontaneously with oxygen in a chemical reaction above a certain temperature. Sugar and salt dissolve in water because their molecular/ionic properties allow this.
Substances tend to be classified in terms of their energy or phase as well as their chemical compositions. The three phases of matter at low energy are Solid, Liquid and Gas.[10] Solids have fixed structures at room temperature which can resist gravity and other weak forces attempting to rearrange them, due to their tight bonds. Liquids have limited bonds, with no structure and flow with gravity. Gases have no bonds and act as free particles. Another way to view the three phases is by volume and shape: roughly speaking, solids have fixed volume and shape, liquids have fixed volume but no fixed shape, and gases have neither fixed volume nor fixed shape.
Water (H2O) is a liquid at room temperature because its molecules are bound by intermolecular forces called Hydrogen bonds. Thus, the forces between the molecules are so large that the energy at room temperature is not high enough to break them. Hydrogen sulfide (H2S) on the other hand is a gas at room temperature and standard pressure, as its molecules are bound by weaker dipole-dipole interactions. The hydrogen bonds in water have enough energy to keep the water molecules from separating from each other but not from sliding around, making it a liquid at temperatures between 0 °C and 100 °C at sea level. Lowering the temperature or energy further, allows for a tighter organization to form, creating a solid, and releasing energy. Increasing the energy (see heat of fusion) will melt the ice although the temperature will not change until all the ice is melted. Increasing the temperature of the water will eventually cause boiling (see heat of vaporization) when there is enough energy to overcome the polar attractions between individual water molecules (100 °C at 1 atmosphere of pressure), allowing the H2O molecules to disperse enough to be a gas. Note that in each case there is energy required to overcome the intermolecular attractions and thus allow the molecules to move away from each other.[11]
Scientists who study chemistry are known as chemists.[12] Most chemists specialize in one or more sub-disciplines. The chemistry taught at the high school or early college level is often called "general chemistry" and is intended to be an introduction to a wide variety of fundamental concepts and to give the student the tools to continue on to more advanced subjects. Many concepts presented at this level are often incomplete and technically inaccurate, yet they are of extraordinary utility. Chemists regularly use these simple, elegant tools and explanations in their work because they have been proven to accurately model a very wide array of chemical reactivity, are generally sufficient, and more precise solutions may be prohibitively difficult to obtain.
The science of chemistry is historically a recent development but has its roots in alchemy which has been practiced for millennia throughout the world.[13]
History

The genesis of chemistry can be traced to the phenomenon of burning which led to metallurgy- the art and science of processing ores to get metals. Later on the science formation of alloys was discovered as a method for strengthening metals. Thus, Gold had been purified long before the first alloys were created, even though, the underlying process for purifying gold was not well understood. It was thought to be a transformation rather than purification. Many scholars in those days thought it reasonable to find a means for transforming cheaper (base) metals into gold. This led to the rise of alchemy, and the search for the Philosopher's Stone, believed to be a potent mean to bring about such a transformation. This development took place over thousands of years.[14]
It is often said that medieval Muslim were the earliest chemists, this claim is particularly based on the works of Geber (d. 815), Al-Kindi (d. 873), Al-Razi (d. 925), and Abu-Rayhan Biruni (d. 1048).[15][16] The works of Geber became more widely known in Europe through Latin translations by a pseudo-Geber in 14th century Spain, who also wrote some of his own books under the pen name "Geber".
One of the main factors that led to the emergence of chemistry in Europe was the recurrent incidence of the plague and blights in Europe during the so called Dark Ages. This gave rise to a need for medicines. It was thought that there might exist a universal medicine that can cure all diseases - called the Elixir of Life. However, like the Philosopher's Stone, neither one were ever found. Modern day chemistry states that such a medicine is not possible. Alchemy for many was an avenue for charlatans to create fake medicines.
For many practitioners of the so called alchemy, it was an intellectual pursuit that could not separate superstition from scientific inquiry. Over time, practitioners got better at it. Paracelsus (1493-1541) rejected the 4-elemental theory and with only a vague understanding of his chemicals and medicines, formed a hybrid of alchemy and science in what was to be called iatrochemistry. The influences of philosophers such as Sir Francis Bacon (1561-1626) and René Descartes (1596-1650), led to a scientific revolution. These philosophers demanded more rigor in mathematics and in removing bias from scientific observations. In chemistry, this began with Robert Boyle (1627-1691), who discovered gases, and came up with equations that were known as Boyle's Law.[17] The person often termed as the Father of Chemistry is Antoine Lavoisier (1743-1794), who developed the theory of Conservation of mass in 1783. Equally important was the development of the Atomic Theory, principally by John Dalton (1766-1844) around 1800.
The discoveries of the chemical elements has a long history from the days of alchemy and culminating in the creation of the periodic table of the chemical elements by Dmitri Mendeleev (1834-1907).[18] The Nobel Prize in Chemistry instituted in 1901 gave a tremendous impetuous to the discovery of new chemical substances and invention of analytical techniques in the past 100 years.
Etymology
The word chemistry comes from the earlier study of alchemy, which is basically the quest to make gold from earthen starting materials.[19] As to the origin of the word "alchemy" the question is a debatable one; it certainly can be traced back to the Greeks, and some, following E. Wallis Budge, have also asserted Egyptian origins. Alchemy, generally, derives from the old French alkemie from the Arabic al-kimia - "the art of transformation". The Arabs borrowed the word "kimia" from the Greeks when they conquered Alexandria in the year 642 AD. A tentative outline is as follows:
- Egyptian alchemy [5,000 BC – 400 BC], formulate early "element" theories such as the Ogdoad.
- Greek alchemy [332 BC – 642 AD], the Greek king Alexander the Great conquers Egypt and founds Alexandria, having the world's largest library, where scholars and "wise" men gather to study.
- Arabian alchemy [642 AD – 1200], the Arabs take over Alexandria; Jabir is the main chemist
- European alchemy [1300 – present], Pseudo-Geber builds on Arabic chemistry
- Chemistry [1661], Boyle writes his classic chemistry text The Sceptical Chymist
- Chemistry [1787], Lavoisier writes his classic Elements of Chemistry
- Chemistry [1803], Dalton publishes his Atomic Theory
Thus, an alchemist was called a 'chemist' in popular speech, and later the suffix "-ry" was added to this to describe the art of the chemist as "chemistry".
Definitions
In retrospect, the definition of chemistry seems to invariably change per decade, as new discoveries and theories add to the functionality of the science. Shown below, for example, are some of the standard definitions used by various noted chemists:
- Alchemy (330) – the study of the composition of waters, movement, growth, embodying and disembodying, drawing the spirits from bodies and bonding the spirits within bodies (Zosimos).[20]
- Chymistry (1661) – the subject of the material principles of mixt bodies (Boyle).[21]
- Chymistry (1663) – a scientifick art, by which one learns to dissolve bodies, and draw from them the different substances on their composition, and how to unite them again, and exalt them to an higher perfection (Glaser).[22]
- Chemistry (1730) – the art of resolving mixt, compound, or aggregate bodies into their principles; and of composing such bodies from those principles (Stahl).[23]
- Chemistry (1837) – the science concerned with the laws and effects of molecular forces (Dumas).[24]
- Chemistry (1947) – the science of substances: their structure, their properties, and the reactions that change them into other substances (Pauling).[25]
- Chemistry (1998) – the study of matter and the changes it undergoes (Chang).[26]
Subdisciplines

Chemistry is typically divided into several major sub-disciplines. There are also several main cross-disciplinary and more specialized fields of chemistry.[27]
- Analytical chemistry is the analysis of material samples to gain an understanding of their chemical composition and structure. Analytical chemistry incorporates standardized experimental methods in chemistry. These methods may be used in all subdisciplines of chemistry, excluding purely theoretical chemistry.
- Biochemistry is the study of the chemicals, chemical reactions and chemical interactions that take place in living organisms. Biochemistry and organic chemistry are closely related, as in medicinal chemistry or neurochemistry. Biochemistry is also associated with molecular biology and genetics.
- Inorganic chemistry is the study of the properties and reactions of inorganic compounds. The distinction between organic and inorganic disciplines is not absolute and there is much overlap, most importantly in the sub-discipline of organometallic chemistry.
- Materials chemistry is the preparation, characterization, and understanding of substances with a useful function. The field is a new breadth of study in graduate programs, and it integrates elements from all classical areas of chemistry with a focus on fundamental issues that are unique to materials. Primary systems of study include the chemistry of condensed phases (solids, liquids, polymers) and interfaces between different phases.
- Nuclear chemistry is the study of how subatomic particles come together and make nuclei. Modern Transmutation is a large component of nuclear chemistry, and the table of nuclides is an important result and tool for this field.
- Organic chemistry is the study of the structure, properties, composition, mechanisms, and reactions of organic compounds. An organic compound is defined as any compound based on a carbon skeleton.
- Physical chemistry is the study of the physical and fundamental basis of chemical systems and processes. In particular, the energetics and dynamics of such systems and processes are of interest to physical chemists. Important areas of study include chemical thermodynamics, chemical kinetics, electrochemistry, statistical mechanics, and spectroscopy. Physical chemistry has large overlap with molecular physics. Physical chemistry involves the use of calculus in deriving equations. It is usually associated with quantum chemistry and theoretical chemistry. Physical chemistry is a distinct discipline from chemical physics.
- Theoretical chemistry is the study of chemistry via fundamental theoretical reasoning (usually within mathematics or physics). In particular the application of quantum mechanics to chemistry is called quantum chemistry. Since the end of the Second World War, the development of computers has allowed a systematic development of computational chemistry, which is the art of developing and applying computer programs for solving chemical problems. Theoretical chemistry has large overlap with (theoretical and experimental) condensed matter physics and molecular physics. Essentially from reductionism theoretical chemistry is just physics, just like fundamental biology is just chemistry and physics.
Other fields include Astrochemistry, Atmospheric chemistry, Chemical Engineering, Chemo-informatics, Electrochemistry, Environmental chemistry, Flow chemistry, Geochemistry, Green chemistry, History of chemistry, Materials science, Medicinal chemistry, Molecular Biology, Molecular genetics, Nanotechnology, Organometallic chemistry, Petrochemistry, Pharmacology, Photochemistry, Phytochemistry, Polymer chemistry, Solid-state chemistry, Sonochemistry, Supramolecular chemistry, Surface chemistry, Immunochemistry and Thermochemistry.
The nature and classifications of matter

Many terms in chemistry have been developed to classify matter.[28]
Atoms
An atom is a collection of matter consisting of a positively charged core (the atomic nucleus) which contains protons and neutrons, and which maintains a number of electrons to balance the positive charge in the nucleus. The Atom is also the smallest portion into which an element can be divided and still retain its properties, made up of a dense, positively charged nucleus surrounded by a system of electrons.
Elements
An element is a class of atoms which have the same number of protons in the nucleus. This number is known as the atomic number of the element. For example, all atoms with 6 protons in their nuclei are atoms of the chemical element carbon, and all atoms with 92 protons in their nuclei are atoms of the element uranium.
The most convenient presentation of the chemical elements is in the periodic table of the chemical elements, which groups elements by atomic number. Due to its ingenious arrangement, groups, or columns, and periods, or rows, of elements in the table either share several chemical properties, or follow a certain trend in characteristics such as atomic radius, electronegativity, etc. Lists of the elements by name, by symbol, and by atomic number are also available. In addition, several isotopes of an element may exist.
Compounds
A compound is a substance with a fixed ratio of chemical elements which determines the composition, and a particular organization which determines chemical properties. For example, water is a compound containing hydrogen and oxygen in the ratio of two to one, with the oxygen between the hydrogens, and an angle of 104.5° between them. Compounds are formed and interconverted by chemical reactions.
Substance
A chemical substance is a general term that can be an element, compound or a mixture of compounds, elements or compounds and elements. Most of the matter we encounter in our daily life are one or another kind of mixtures, e.g. air, alloys, biomass etc.
Molecules
A molecule is the smallest indivisible portion of a pure compound or element that retains a set of unique chemical properties. Molecules differ from other chemical entities in that they can and often do exist as single electrically neutral units. Salts, for example, do not consist of molecular units but rather of many cations and anions in a crystal lattice. Molecules are typically a set of atoms bound together by covalent bonds, such that the structure is electrically neutral and all valence electrons are paired with other electrons either in bonds or in lone pairs.
Ions and Salts
An ion is a charged species, or an atom or a molecule that has lost or gained one or more electrons. Positively charged cations (e.g. sodium cation Na+) and negatively charged anions (e.g. chloride Cl−) can form neutral salts (e.g. sodium chloride NaCl). Examples of polyatomic ions that do not split up during acid-base reactions are hydroxide (OH−) and phosphate (PO43−).
States of matter
In addition to the specific chemical properties that distinguish different chemical classifications chemicals can exist in several phases. For the most part, the chemical classifications are independent of these bulk phase classifications; however, some more exotic phases are incompatible with certain chemical properties. A phase is a set of states of a chemical system that have similar bulk structural properties, over a range of conditions, such as pressure or temperature. Physical properties, such as density and refractive index tend to fall within values characteristic of the phase. The phase of matter is defined by the phase transition, which is when energy put into or taken out of the system goes into rearranging the structure of the system, instead of changing the bulk conditions.
Sometimes the distinction between phases can be continuous instead of having a discrete boundary, in this case the matter is considered to be in a supercritical state. When three states meet based on the conditions, it is known as a triple point and since this is invariant, it is a convenient way to define a set of conditions.
The most familiar examples of phases are solids, liquids, and gases. Many substances exhibit multiple solid phases. For example, there are three phases of solid iron (alpha, gamma, and delta) that vary based on temperature and pressure. A principle difference between solid phases is the crystal structure, or arrangement, of the atoms. Less familiar phases include plasmas, Bose-Einstein condensates and fermionic condensates and the paramagnetic and ferromagnetic phases of magnetic materials. While most familiar phases deal with three-dimensional systems, it is also possible to define analogs in two-dimensional systems, which has received attention for its relevance to systems in biology.
Fundamental concepts and theories
Nomenclature
The standard nomenclature is set by the International Union of Pure and Applied Chemistry (IUPAC). Nomenclature refers to a system for naming chemical compounds. There are well-defined systems in place for naming chemical species. Organic compounds are named according to the organic nomenclature system.[29] Inorganic compounds are named according to the inorganic nomenclature system.[30] Nomenclature is a critical part of the language of chemistry and the IUPAC system of chemical nomenclature used today allows chemists to specify by name specific compounds amongst the infinite variety of possible chemicals.
Chemical reactions
A Chemical reaction is a process that results in the interconversion of chemical substances. Such reactions can result in molecules combining to form larger molecules, molecules breaking apart to form two or more smaller molecules, or rearrangement of atoms within or across molecules. Chemical reactions usually involve the making or breaking of chemical bonds. For example, substances that react with oxygen to produce other substances are said to undergo oxidation; similarly a group of substances called acids or alkalis can react with one another to neutralize each other's effect, a phenomenon known as neutralization. Substances can also be dissociated or synthesized from other substances by various different chemical processes.
A stricter definition exists[31] that states "a Chemical Reaction is a process that results in the interconversion of chemical species". Under this definition, a chemical reaction may be an elementary reaction or a stepwise reaction. An additional caveat is made, in that this definition includes cases where the interconversion of conformers is experimentally observable. Such detectable chemical reactions normally involve sets of molecular entities as indicated by this definition, but it is often conceptually convenient to use the term also for changes involving single molecular entities (i.e. 'microscopic chemical events').
Chemical energetics
A chemical reaction invariably absorbs or releases energy in the form of heat or light. Usually there is an energy barrier between the initial state of reactants, and final state of products of a chemical reaction. Thus, a chemical reaction is invariably not possible unless the reactants surmount that barrier known as the activation energy. This energy that is necessary for a chemical reaction can be in the form of heat, light, electricity or mechanical force in the form of ultrasound[32]. A reaction is said to be exothermic if the final state is lower on the energy scale than the initial state; in case of endothermic reactions the situation is otherwise.
The possible states of energy of electrons, atoms and molecules is determined by the rules of quantum mechanics, which require quantization of energy of a bound system. The atoms and/or molecules of a chemical substance can exist in several energy states. The atoms/molecules in an higher energy state are said to be excited. The molecules/atoms of substance in an excited energy state are often much more reactive, that is amenable to chemical reactions. Thus, the speed of a chemical reaction (at given temperature T) is related to the activation energy E, by the Boltzmann's population factor - that is the probability of molecule to have energy greater than or equal to E at the given temperature T. This exponential dependence of a reaction rate on temperature is known in chemistry as the Arrhenius equation.
Several characteristic quanta of energy enable the transfer of energy from one chemical substance to other. Energy is transferable from one substance to another only if the size of energy quanta emitted from one substance matches with the energy levels of the other. Heat energy is easily transferred from almost any substance to another mainly because energy levels due to vibrations of atoms and molecules are very closely placed, same is the case with rotational energy levels. However, the electronic energy levels are not so closely spaced, hence ultraviolet electromagnetic radiation is not transferred with equal felicity, as is also the case with electrical energy.
The existence of characteristic energy levels for different chemical substances is also useful for their identification by the analysis of spectral lines of different kinds of spectra often used in chemical spectroscopy e.g. IR, microwave, NMR, ESR etc. This is used to identify the composition of remote objects - like stars and far galaxies - by analyzing their radiation (see spectroscopy).

These lines (so called because they appear as linear features in dispersion spectra (see example above), such as might be produced by a prism or diffraction grating) are the results of release or absorption of certain specific amount of energy involved in the transition of atoms or molecules from one state to another.
Chemical Energy
The potential of a chemical substance to undergo a transformation through a chemical reaction or transform other chemical substances is often referred to as its chemical energy. Since energy is involved in breaking or making of chemical bonds, it is released as a result of a chemical reaction in case the energy of the reactants is more than that in products, otherwise it is absorbed.
The energy that can be released (or absorbed) because of a reaction between a set of chemical substances is equal to the difference between the energy content of the product substances and that of the reactants.This change in energy is rigorously the change in internal energy. It can be calculated using the formula
ΔUo = Σ(ΔUfoproducts) - Σ(ΔUforeactants).
Where ΔUforeactants is the internal energy of formation of the reactant molecules that can be calculated from the bond energies of the various chemical bonds of the molecules under consideration and ΔUfoproducts is the internal energy of formation of the product molecules. The internal energy change of a process is equal to the heat change if it is measured under conditions of constant volume, as in a closed rigid container such as a bomb calorimeter. Under conditions of constant pressure, as in reactions in vessels open to the atmosphere, the heat change measured is not always the same as the internal energy change, since pressure-volume work releases or absorbs heat and energy. (The heat change at constant pressure is called the enthalpy change, in this case the enthalpy of formation).
The chemical potential energy of a fuel appears as its heat of combustion. Food is similar to hydrocarbon fuel and carbohydrate fuels, and when it is oxidized, its caloric content is similar (though not assessed in the same way as a hydrocarbon fuel-- see food energy).
In chemical thermodynamics the term used for the chemical potential energy is chemical potential and for chemical transformation an equation most often used is Gibbs-Duhem equation
Chemical laws
The most fundamental concept in chemistry is the law of conservation of mass, which states that there is no detectable change in the quantity of matter during an ordinary chemical reaction.[33] Modern physics shows that it is actually energy that is conserved, and that energy and mass are related; a concept which becomes important in nuclear chemistry. Conservation of energy leads to the important concepts of equilibrium, thermodynamics, and kinetics.
Further laws of chemistry elaborate on the law of conservation of mass. Joseph Proust's law of definite composition says that pure chemicals are composed of elements in a definite formulation; we now know that the structural arrangement of these elements is also important.
Dalton's law of multiple proportions says that these chemicals will present themselves in proportions that are small whole numbers (i.e. 1:2 O:H in water); although in many systems (notably biomacromolecules and minerals) the ratios tend to require large numbers, and are frequently represented as a fraction. Such compounds are known as non-stoichiometric compounds.
Bonding
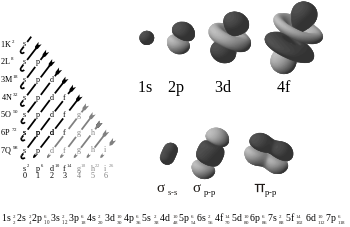
A chemical bond is the multipole balance between the positive charges in the nuclei and the negative charges oscillating about them.[34] More than simple attraction and repulsion, the energies and distributions characterize the availability of an electron to bond to another atom. These potentials create the interactions which holds together atoms in molecules or crystals. In many simple compounds, Valence Bond Theory, the Valence Shell Electron Pair Repulsion model (VSEPR), and the concept of oxidation number can be used to predict molecular structure and composition. Similarly, theories from classical physics can be used to predict many ionic structures. With more complicated compounds, such as metal complexes, valence bond theory fails and alternative approaches, primarily based on principles of quantum chemistry such as the molecular orbital theory, are necessary. See diagram on electronic orbitals.
Quantum chemistry
Quantum chemistry mathematically describes the fundamental behavior of matter at the molecular scale.[35] It is, in principle, possible to describe all chemical systems using this theory. In practice, only the simplest chemical systems may realistically be investigated in purely quantum mechanical terms, and approximations must be made for most practical purposes (e.g., Hartree-Fock, post Hartree-Fock or Density functional theory, see computational chemistry for more details). Hence a detailed understanding of quantum mechanics is not necessary for most chemistry, as the important implications of the theory (principally the orbital approximation) can be understood and applied in simpler terms.
In quantum mechanics (several applications in computational chemistry and quantum chemistry), the Hamiltonian, or the physical state, of a particle can be expressed as the sum of two operators, one corresponding to kinetic energy and the other to potential energy. The Hamiltonian in the Schrödinger wave equation used in quantum chemistry does not contain terms for the spin of the electron.
Solutions of the Schrödinger equation for the hydrogen atom gives the form of the wave function for atomic orbitals, and the relative energy of say the 1s,2s,2p and 3s orbitals. The orbital approximation can be used to understand the other atoms e.g. helium, lithium and carbon.
Chemical industry
The chemical industry represents an important economic activity. The global top 50 chemical producers in 2004 had sales of 587 billion US dollars with a profit margin of 8.1% and research and development spending of 2.1% of total chemical sales.[36]
See also
Lists
- Common chemicals - Where to find common chemical components
- List of basic chemistry topics
- List of chemistry topics
- List of chemists
- List of compounds
- List of important publications in chemistry
- Periodic Table of the Elements
- Timeline of chemistry
- Unsolved problems in chemistry
Related topics
- Alchemy
- Biochemistry
- Chemical engineering
- History of chemistry
- Linus Pauling
- Registration, Evaluation and Authorisation of Chemicals - A proposed European Union regulation
- Perfection ("Perfection in physics and chemistry")
References
- ^ See: Chemistry (etymology) for possible origins of this word.
- ^ Mi Gyung, Kim (2003). Affinity, That Elusive Dream - A Genealogy of the Chemical Revolution. MIT Press. ISBN 0-262-11273-6.
- ^ [A] John Warren (2005). "War and the Cultural Heritage of Iraq: a sadly mismanaged affair", Third World Quarterly, Volume 26, Issue 4 & 5, p. 815-830.
[B] Dr. A. Zahoor (1997). JABIR IBN HAIYAN (Geber). University of Indonesia.
[C] Paul Vallely. How Islamic inventors changed the world. The Independent. - ^ Theodore L. Brown, H. Eugene Lemay, Bruce Edward Bursten, H. Lemay. Chemistry: The Central Science. Prentice Hall; 8 edition (1999). ISBN 0130103101. Pages 3-4.
- ^ Chemistry has also been called the central science because it is seen as occupying an intermediate position in a hierarchy of the sciences by "reductive level", between physics and biology. See Carsten Reinhardt. Chemical Sciences in the 20th Century: Bridging Boundaries. Wiley-VCH, 2001. ISBN 3527302719. Pages 1-2.
- ^ IUPAC Gold Book Definition
- ^ Hydrogen Fuel Cells [1]
- ^ What is Chemistry?[2]
- ^ Matter: Atoms from Democritus to Dalton by Anthony Carpi, Ph.D.[3]
- ^ Chem4Kids.com: Matter: States of Matter [4]
- ^ Chem4Kids.com: Changing states of matter[5]
- ^ California Occupational Guide Number 22: Chemists[6]
- ^ Dictionary of the History of Ideas: Alchemy [7]
- ^ Chemical Heritage Foundation: Ancients and Alchemists [8]
- ^ Will Durant (1980), The Age of Faith (The Story of Civilization, Volume 4), p. 162-186, Simon & Schuster, ISBN 0671012002:
"Chemistry as a science was almost created by the Muslims; for in this field, where the Greeks (so far as we know) were confined to industrial experience and vague hypothesis, the Saracens introduced precise observation, controlled experiment, and careful records. They invented and named the alembic (al-anbiq), chemically analyzed innumerable substances, composed lapidaries, distinguished alkalis and acids, investigated their affinities, studied and manufactured hundreds of drugs. Alchemy, which the Muslims inherited from Egypt, contributed to chemistry by a thousand incidental discoveries, and by its method, which was the most scientific of all medieval operations."
- ^ Dr. K. Ajram (1992), Miracle of Islamic Science, Appendix B, Knowledge House Publishers, ISBN 0911119434:
"Humboldt regards the Muslims as the founders of chemistry."
- ^ BBC - History - Robert Boyle (1627 - 1691) [9]
- ^ About: Chemistry - Time line of Element Discovery [10].
- ^ Alchemy Lab: History of Alchemy [11]
- ^ Strathern, P. (2000). Mendeleyev’s Dream – the Quest for the Elements. New York: Berkley Books.
- ^ Boyle, Robert (1661). The Sceptical Chymist. New York: Dover Publications, Inc. (reprint). ISBN 0486428257.
- ^ Glaser, Christopher (1663). Traite de la chymie. Paris. as found in: Kim, Mi Gyung (2003). Affinity, That Elusive Dream - A Geanealogy of the Chemical Revolution. The MIT Press. ISBN 0-262-11273-6.
- ^ Stahl, George, E. (1730). Philosophical Principles of Universal Chemistry. London.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Dumas, J. B. (1837). 'Affinite' (lecture notes), vii, pg 4. “Statique chimique”, Paris: Academie des Sciences
- ^ Pauling, Linus (1947). General Chemistry. Dover Publications, Inc. ISBN 0486656225.
- ^ Chang, Raymond (1998). Chemistry, 6th Ed. New York: McGraw Hill. ISBN 0-07-115221-0.
- ^ The Canadian Encyclopedia: Chemistry Subdisciplines [12]
- ^ General Chemistry Online - Companion Notes: Matter [13]
- ^ IUPAC Nomenclature of Organic Chemistry [14]
- ^ IUPAC Provisional Recommendations for the Nomenclature of Inorganic Chemistry (2004) [15]
- ^ Gold Book Link
- ^ http://www.newscientisttech.com/article/dn11427
- ^ Fundamental laws of chemical reactions and chemical equation [16]
- ^ visionlearning: Chemical Bonding by Anthony Carpi, Ph. [17]
- ^ Quantum Chemistry [18]
- ^ "Top 50 Chemical Producers". Chemical & Engineering News. 83 (29): 20–23. July 18, 2005.
{{cite journal}}: Check date values in:|date=(help)
Further reading
Popular reading
- Atkins, P.W. Galileo's Finger (Oxford University Press) ISBN 0198609418
- Atkins, P.W. Atkins' Molecules (Cambridge University Press) ISBN 0521823978
- Stwertka, A. A Guide to the Elements (Oxford University Press) ISBN 0195150279
Introductory undergraduate text books
- Chang, Raymond. Chemistry 6th ed. Boston: James M. Smith, 1998. ISBN 0-07-115221-0.
- Atkins, P.W., Overton, T., Rourke, J., Weller, M. and Armstrong, F. Shriver and Atkins inorganic chemistry (4th edition) 2006 (Oxford University Press) ISBN 0-19-926463-5
- Clayden, J., Greeves, N., Warren, S., Wothers, P. Organic Chemistry 2000 (Oxford University Press) ISBN 0-19-850346-6
- Voet and Voet Biochemistry (Wiley) ISBN 0-471-58651-X
Advanced Undergraduate-level or Graduate text books
- Atkins, P.W. Physical Chemistry (Oxford University Press) ISBN 0-19-879285-9
- Atkins, P.W. et al. Molecular Quantum Mechanics (Oxford University Press)
- McWeeny, R. Coulson's Valence (Oxford Science Publications) ISBN 0-19-855144-4
- Pauling, L. The Nature of the chemical bond (Cornell University Press) ISBN 0-8014-0333-2
- Pauling, L., and Wilson, E. B. Introduction to Quantum Mechanics with Applications to Chemistry (Dover Publications) ISBN 0-486-64871-0
- Stephenson, G. Mathematical Methods for Science Students (Longman)ISBN 0-582-44416-0
- Smart and Moore Solid State Chemistry: An Introduction (Chapman and Hall) ISBN 0-412-40040-5
Professional societies
- American Chemical Society
- Chemical Institute of Canada
- Chemical Society of Peru
- International Union of Pure and Applied Chemistry
- Royal Australian Chemical Institute
- Royal Society of Chemistry
- Society of Chemical Industry
- World Association of Theoretical and Computational Chemists
External links
- International Union of Pure and Applied Chemistry
- IUPAC Nomenclature Home Page, see especially the "Gold Book" containing definitions of standard chemical terms
- Interactive Mind Map of Chemistry
- / Chemical energetics
For a full list of external links and suppliers see Wikipedia:Chemical sources


