Shale oil extraction

Oil shale extraction is an industrial process in which kerogen—a mixture of organic chemical compounds (including hydrocarbons) found in oil shale—is converted into synthetic crude oil through pyrolysis. In pyrolysis, oil shale is heated in the absence of oxygen until its kerogen decomposes into a petroleum-like condensable shale oil—a form of non-conventional oil—and combustible shale gas (shale gas can also refer to the gases that occur naturally in shales). In addition, oil shale extraction produces a solid residue—spent shale (char). The decomposition of oil shale begins at 300 °C (570 °F), but proceeds more rapidly and completely at higher temperatures.[1]
Oil shale pyrolysis is commonly performed above ground (ex-situ processing), although some newer technologies perform the process underground (on-site or in-situ processing).[2] A number of oil shale pyrolysis and retorting technologies have been patented. While a few dozen have been tested in a pilot plant (with a capacity between 1 and 10 tonnes of oil shale per hour), fewer than ten technologies are known to have been tested at a demonstration scale (40 to 400 tonnes per hour). As of 2008, only four technologies are in commercial use: Kiviter, Galoter, Fushun, and Petrosix.[3]
Pyrolysis is endothermic; it requires an external source of energy. Most technologies use combustion of the shale or other fuels to generate heat, although some experimental extraction methods use electricity, radio frequency and microwaves, and reactive fluids for this purpose.[2] Non-condensible retort shale gas and char may be burned as an additional source of energy, and the heat of the spent shale may be reused to pre-heat the raw oil shale. A variety of direct and indirect methods are used for heat transfer.[1] Almost all of the commercial retorts currently in operation or under development are internal heating retorts.[3]
Oil shale has gained attention as an energy resource due to rising prices of conventional hydrocarbons such as petroleum.[4] By 2008, oil shale extraction was being undertaken in Estonia, Brazil and China, while Australia, USA, Canada and Jordan have planned to start or restore shale oil production.[5][6] The shale oil may be used as a fuel oil or upgraded to meet refinery feedstock specifications by adding hydrogen and removing impurities such as sulfur and nitrogen.
Oil shale processing involves a number of environmental management issues, such as waste disposal, extensive water use and waste water management, and air pollution.[7]
Classifications
Industry analysts have created several classifications of the methods by which hydrocarbons are extracted from oil shale.
By location
A frequently-used distinction considers whether retorting is done above or below ground, and classifies the technologies broadly as ex situ (displaced) or in situ (in place). In the ex situ process, also known as above-ground retorting, the oil shale is mined and transported to a retort facility. In contrast, the in situ method converts the kerogen while it is still in the form of an oil shale deposit; it is then extracted via an oil well, where it rises in the same way as conventional petroleum.
By retort style
Based on the materials and techniques used to heat the oil shale to an appropriate temperature, its processing has been classified into internal combustion, hot recycled solids, wall conduction, externally-generated hot gas, reactive fluid, and volumetric heating methods. There are many possible realizations and combinations of these methods; therefore the following table is representative but not complete. Some processes are difficult to classify due to their unique methods of heat input (e.g. ExxonMobil's Electrofrac) or due to limited information.[2]
| Heating Method | Above Ground (ex situ) | Below Ground (in situ) |
|---|---|---|
| Internal combustion | Kiviter, Fushun, Union A, Paraho Direct, Superior Direct | Oxy MIS, LLNL RISE, Geokinetics Horizontal, Rio Blanco |
| Hot recycled solids (inert or burned shale) |
Alberta Taciuk, Galoter, Lurgi, TOSCO II, Chevron STB, LLNL HRS, Shell Spher | - |
| Conduction through a wall (various fuels) |
Pumpherston, Hom Tov, Fischer Assay, Oil-Tech, EcoShale In-Capsule Process, Combustion Resources | Shell ICP (primary method), EGL Oil Shale Process, IEP Geothermic Fuel Cell Process |
| Externally generated hot gas | PetroSIX, Union B, Paraho Indirect, Superior Indirect, Syntec process (Smith process) | Chevron CRUSH, Petro Probe, MWE IGE |
| Reactive fluids | IGT Hytort (high-pressure H2), Xtract Technology (supercritical solvent extraction), Donor solvent processes, Chattanooga fluidized bed reactor | Shell ICP (some embodiments) |
| Volumetric heating | - | ITTRI, LLNL and Raytheon radiofrequency processes, Global Resource microwave process, Electro-Petroleum EEOP |
The heating methods used to decompose the rock may be classified as direct or indirect. Internal combustion technologies, which burn materials within the retort, are classified as direct - all other heating technologies are described as indirect. Another scheme considers whether gases or circulated solids are used to transfer heat, and distinguishes between internal hot gas carrier technologies (e.g. internal combustion technologies and externally-generated hot gas technologies) and internal hot solid carrier technologies (those using hot recycled solids to transfer heat to the oil shale and those which conduct heat to the retort through a wall).[1][3][2]
Some analysts use the size of the oil shale particles that are fed into the retorts to differentiate the various ex situ extraction processes. As a rule, oil shale "lumps" varying in diameter from 10 to 100 millimetres (0.4 to 4 in) are used in internal hot gas carrier technologies, while oil shale that has been crushed into particulates less than 10 millimetres (0.4 in)* in diameter is used in internal hot solid carrier technologies.[3]
Ex situ technologies
In ex situ methods, the oil shale is mined either underground or at the surface and then transported to a processing facility. At the facility, the oil shale is heated, usually to between 450 °C and 500 °C (840 °F to 930 °F). At these temperatures the kerogen in the oil shale decomposes into gas, oil vapor, and char. This process is known as retorting. The gas and oil vapors are separated from the spent shale and cooled, causing the oil to condense.
Internal combustion
Internal combustion technologies burn materials within the retort to supply heat; the retort vapors mix with the exhaust generated by combustion. Lump oil shale is used as a feed. The principal technologies in this category are the Kiviter, Union A, Paraho Direct, Superior Direct, and Fushun processes.[2][8]
In the Kiviter process, shale within a vertical shaft retort is brought to temperature by igniting coke residue (char) and non-condensable shale gas. Raw oil shale is fed into the top of the retort, and is heated by the rising combustion gases below it, which pass laterally through the descending oil shale. The shale oil vapors and evolving gases are directed into a collection chamber. After entering the collection chamber, the hot oil vapor is delivered to a condensing system. The shale residue is heated to about 900 °C (1,650 °F) to burn off the char. Recycle gas enters the bottom of the retort and cools the spent shale, which then leaves the retort through a water-sealed discharge system.[1] There are environmental issues associated with the Kiviter process; it uses large amounts of water, which is polluted during processing, and the solid waste residue contains water-soluble toxic substances that leach into the surrounding area.[9][10] The Kiviter process is used by the Estonian Viru Keemia Grupp's subsidiary VKG Oil.[11] The company operates several Kiviter retorts, the largest of them having a processing capacity of 40 tonnes per hour of oil shale feedstock.
The Fushun process uses a vertical shaft retort similar to the Kiviter retort. The Fushun Mining Group in Liaoning Province, China, operates the largest shale oil plant in the world. In 2003, the group operated 80 Fushun retorts; by 2007 it was operating 180. Each retort processes about 4 tonnes of shale per hour.[12]
The Paraho Direct is a US version of a vertical shaft kiln.[13] This technology is used by Shale Technologies LLC in a pilot plant facility in Rifle, Colorado.[14][15][16]
Hot recycled solids
Hot recycled solids technologies use solid particles - usually shale ash - to carry heat in the retorting zone. The principal technologies in this category include the Galoter, Alberta Taciuk, TOSCO II, Lurgi-Ruhrgas, Chevron STB, LLNL HRS, and Shell Spher processes. Typically, these technologies use a rotating kiln, fed by fine oil shale particles. The particles are generally smaller than 10 mm in diameter; some technologies use particles even smaller than 2.5 mm. The particles are heated in a separate chamber or vessel, preventing the dilution of product gas with combustion exhaust.[2][8]
In the Galoter (also known as TSK or SHC) process, spent shale is burnt in a separate furnace. The resulting hot (800 °C (1,470 °F)) ash is mixed with dried oil shale and used as the heat carrier.[10] The temperature of pyrolyis in the Galoter retort is 520 °C (970 °F).[1] This process creates less pollution than internal combustion technologies, as it uses less water, but carbon dioxide is still generated and the burning residue produces organic carbon disulfide and calcium sulfide.[9] Two Galoter installations are used for oil production by Eesti Energia, an Estonian energy company.[11] Both process 125 tonnes per hour of oil shale. The company plans to construct two additional units in Estonia, beginning in 2009. In addition, Eesti Energia has made plans to build a Galoter plant in Jordan; the construction is slated to begin by 2015.[17][18][19] VKG Oil is considering construction of a Galoter process plant engineered by Atomenergoproject of Saint Petersburg.[20]
The Alberta Taciuk is similar to the Galoter process in that heat transfer is performed by fine particles of oil shale in a rotating kiln. Its distinguishing feature is that the drying and pyrolysis of the feed shale and the combustion, recycling, and cooling of spent shale all occur in a single rotating multi-chamber horizontal vessel.[3][21][22] The water pollution caused by this process is quite limited.[9] Two Australian oil companies, Southern Pacific Petroleum NL and later Queensland Energy Resources, operated a 250 tonnes per hour Alberta Taciuk Processor industrial-scale pilot plant which was shut down in 2004.[3][22] UMATAC Industrial Processes designed a 250 tonnes per hour ATP retort in China, which was scheduled to be operational by 2008.[23] According to Jordan Energy and Mining Ltd, the company plans to use this technology for its project in Jordan.[19][24]
Two other major technologies employ hot solids. As in the Galoter and Alberta Taciuk processes, TOSCO II uses fine particles of oil shale that are heated in a rotating kiln. Instead of recycling shale ash, however, TOSCO II circulates hot ceramic balls between the retort and a heater. The process was tested in a 40 tonnes-per-hour test facility near Parachute, Colorado; this facility was, however, shut down in 1972. Lawrence Livermore National Laboratory developed the hot recycled solid (LLNL HRS) retorting process. This technology was used in a 4 tonnes-per-day pilot plant between 1990 and 1993. The delayed-fall combustor used in this process gives greater control over the combustion process as compared to a lift pipe combustor, used in the older Lurgi-Ruhrgas process, originally developed during the 1950s in Germany by Lurgi Corporation. Another difference is that the LLNL HRS process uses a fluidized bed mixer instead of the screw mixer used in the Lurgi-Ruhrgas process.[2][11][25] In the LLNL HRS process, most of the pyrolysis occurs in a settling-bed unit (plug flow).[2]
Conduction through a wall
A variety of technologies, including the Fischer Assay, Pumpherston, Hom Tov, and Oil-Tech processes, transfer heat to the oil shale by conducting it through the retort wall. These technologies generally use fine particles; their advantage lies in the fact that retort vapors are not combined with combustion exhaust.[2][8] The Fischer Assay is a standardized laboratory test used to measure the grade of an oil shale sample. A 100 gram sample crushed to <2.38 mm is heated in a small aluminum retort to 500 °C (930 °F) at a rate of 12 °C (54 °F) per minute, and held at that temperature for 40 minutes.[26] The distilled vapors of oil, gas, and water are passed through a condenser and cooled with ice water into a graduated centrifuge tube. The oil yields achieved by other technologies are often reported as a percentage of the Fischer Assay oil yield.
In the Hom Tov process, fine particles of oil shale are slurried with waste bitumen and pumped through coils in a heater. The promoters of this process assert that the technology enables the shale to be processed at somewhat lower temperatures, since the bitumen acts as a catalyst. The technology has not yet been demonstrated in a pilot plant.[27]
In the first step of the Red-Leaf Resources EcoShale In-Capsule Process, a hot gas is generated by burning natural gas or pyrolysis gas. It is then circulated through oil shale rubble using a set of parallel pipes. The heat is transferred to the shale through the pipe walls rather than being injected directly into the rubble, thereby avoiding dilution of the product gas with the heating gas. The pile of oil shale rubble is enclosed by a low-cost earthen impoundment structure designed to prevent environmental contamination and to provide easy reclamation. Heat from the spent shale is recovered, enhancing the process's energy efficiency, by passing cool gas through pipes and then using it to preheat adjacent capsules.[28][29]
A new process from Combustion Processes, Inc., seeks to eliminate carbon dioxide emissions from the shale oil production process. Pyrolysis occurs in a rotating kiln heated by hot gas flowing through an outer annulus. The hot gas is created by burning hydrogen generated in a separate unit by coal gasification followed by carbon dioxide separation. The geometry of the annulus enables the transfer of heat to the moving shale through a wall.[30]
The Oil-Tech staged electrically heated retort process was developed by Millennium Synfuels, LLC (former Oil Tech Inc.). In this process, crushed oil shale is lifted by a conveyor system to the vertical retort, and is loaded into the retort from the top. The retort consists of a series of connected individual heating chambers, stacked atop each other. Heating rods extend into the centers of each of these chambers. The feed oil shale is heated to increasingly-higher temperatures as it moves down the retort, attaining a temperature of 1,000 °F (540 °C) in the lowest chamber. The gases and vapors are vacuumed into a condensing unit. The spent shale is used for pre-heating feed oil shale.[28][31] The advantages of this technology are its modular design, which enhances its portability and adaptability, its low water requirements, its heating efficiency, and the relatively high quality of the resulting product.[31]
Externally generated hot gas
Externally generated hot gas technologies or indirectly heated technologies use heat, transferred by gases which are heated outside the retort vessel. The main technologies are Petrosix, Union B, Paraho Indirect, and Superior Indirect processes.[2][8] As with the internal combustion technologies, most of the externally-generated hot gas technologies process oil shale lumps in vertical shaft kilns; however, the retort vapors are not diluted with combustion exhaust. The world’s largest operational surface oil shale pyrolysis reactor is the Petrosix which is located in São Mateus do Sul, Paraná, Brazil. The 11 metres (36 ft) diameter vertical shaft kiln is owned by Petrobras and has been operating since 1992 with high availability. The company operates two retorts, the largest of which processes 260 tonnes per hour of oil shale.[11][32] Oil Shale Exploration Company LLC intends to use the Petrosix process as the technology to process the mined oil shale into shale oil at the White River Mine near Vernal, Utah.[33][34]
The largest retort ever built used the Union B technology, developed by Unocal. The Union B processed 400 tonnes per hour of oil shale lumps heated by externally generated hot gas. However, unlike all other vertical shaft kilns, the Union B pumped the oil shale into the bottom of the retort, with the hot gas entering at the top. Unocal operated the retort from 1986 to 1992 near Parachute, Colorado. The Paraho Indirect technology is similar to the Petrosix which is considered a highly reliable technology for use with U.S. oil shale.[11]
Two companies, Syntec Energy and Western Energy Partners, have proposed new hot gas processes based on linking coal gasification with rotating kiln retorts. The hot, hydrogen-rich synthesis gas from the coal gasifier is fed into the rotating kiln in direct contact with the oil shale, thereby heating it to pyrolysis temperature. The effluent synthesis gas is then used to generate electric power or other products typical of synthesis gas processes.[28]
Reactive fluids
Reactive fluids technologies include the IGT Hytort (high-pressure H2) process, Xtract Technology (supercritical solvent extraction), donor solvent processes, and the Chattanooga fluidized bed reactor.[2][35][28] In the IGT Hytort process, developed by the Institute of Gas Technology (IGT), oil shales are processed at controlled heating rates in a high-pressure hydrogen environment.[36] This technology, as with other reactive fluid technologies, is useful for processing oil shales with a low hydrogen content, such as the Eastern US Devonian formations. In these shales, only a third of the organic carbon is typically converted to oil during conventional above-ground retorting. Hydrogen or hydrogen donors (chemicals that donate hydrogen during chemical reactions) react with coke precursors (a chemical structure in the oil shale that is prone to form coke during retorting but has not yet done so). The reaction roughly doubles the yield of oil, depending on the characteristics of the shale and process.[37]
Chattanooga Corporation has developed an extraction process that uses a fluidized bed reactor and an associated hydrogen-fired heater. In this process, retorting occurs at relatively low temperatures (1,000 °F (540 °C)*) through thermal cracking and hydrogenation of the shale into hydrocarbon vapors and spent solids. The thermal cracking allows hydrocarbon vapors to be extracted and scrubbed of solids. The vapors are then cooled, inducing the condensate to drop out of the gas. The remaining hydrogen, light hydrocarbons, and acid gases are passed through an amine scrubbing system to remove hydrogen sulfide which is converted to elemental sulfur. The cleaned hydrogen and light hydrocarbon gases are then fed back into the system for compression or into the hydrogen heater to provide heat for the fluidized bed reactor. This system is a nearly-closed loop; almost all of its energy needs are provided by the source material. The demonstration plant in Alberta was able to produce 930 barrels (~130 t) of oil per kilotonne of oil shale with an API gravity ranging between 28 and 30. With hydrotreating (the reaction of oil with high pressure hydrogen), it would be possible to improve this to 38-40 °API. Chattanooga Corporation is considering a design that would implemented in a 2,500-barrel-per-hour (~330 t/h) facility.[28]
In situ technologies
In situ processes heat the oil shale underground. These technologies are potentially able to extract more oil from a given area of land than conventional ex situ processing technologies, as the wells can reach greater depths than surface mines.[38] They present an opportunity to recover shale oil from low-grade deposits that would not be reachable by ordinary mining techniques.[39] Several companies have patented methods for in situ retorting. However, most of these methods are still in experimental stages.
These technologies are usually classified as true in situ processes (TIS) and modified in situ processes (MIS). True in situ processes do not involve mining or crushing the oil shale. Modified in situ processes involve drilling and fracturing the target deposit to create voids occupying between 20 and 25% of the area. The voids improve the flow of gases and fluids through the rock, thereby increasing the volume and quality of the oil produced.[11]
Early in situ processes
A variety of in situ processes were tried before the United States oil shale industry crashed in the 1980s. Most notable are the Equity Oil process, which injected superheated steam into the permeable leached zone of Colorado's Piceance Basin,[40] and the Geokinetics Process. The latter used a horizontal combustion retort; the formation was made more permeable by explosive uplift and the creation of variously-sized fragments (rubblization).[41][42] Little is known of the yields from the Equity process, but the Geokinetics process generally recovered 40-50% of the Fischer Assay oil.[41]
Variations of the modified in situ (MIS) process have been investigated by the US Bureau of Mines, Lawrence Livermore National Laboratory, Occidental Petroleum, Rio Blanco Corporation, and Multi-Mineral Corporation. A proposal in the 1960s involved the creation of a rubble chimney using a nuclear explosive.[43] However, this approach was abandoned for a number of technical reasons. Subsequently, a variety of rubblization approaches were explored. The first MIS oil shale experiment was conducted by Occidental Petroleum in 1972 at Logan, Washington.[11] The installation's oil yield was adversely affected by non-uniform permeability in the formation, causing an inefficient sweep of the rubblized zone.[44] A later series of field experiments aimed at creating a more uniform permeable zone were carried out using different mining and blasting techniques. Depending on the calculation used, Occidental achieved 50-60% Fischer Assay oil yield in Retorts 7 and 8.[45] The Rio Blanco Corporation used a mining and blasting approach that created a bed with close to 40% porosity. This enabled them to retort the chimney at a substantially faster rate and achieve higher oil yields (about 70% of the Fischer Assay).[46] The Multi-Mineral Corporation proposed a more complex MIS process for Saline Zone oil shale; it included recovery of the minerals nahcolite and dawsonite.[47][48]
Modern in situ processes
There has been a recent resurgence of interest in in situ recovery processes. The processes either inject hot fluids into the formation (Chevron CRUSH and Petro Probe) or use line or plane heating sources following by thermal conduction and convection to distribute heat through the formation (Shell ICP, EGL Resources, and ExxonMobil Electrofrac). Most of these processes are expected to produce an oil with an API gravity of about 40 containing fewer olefins and polar molecules due to in situ oil coking and cracking.
Wall conduction
In situ wall conduction technologies include Shell's In situ Conversion Process (ICP), the EGL Resources Process, and the IEP Geothermic Fuels Cells Process. Since 2000, the Shell Oil Company has been developing its in situ method at the Mahogany Research Project, located 200 miles (320 km) west of Denver on Colorado's Western Slope. Although this method is energy-intensive, it compares well to other heavy oil projects such as tar sands development. Over the project life cycle, Shell estimates that for every unit of energy consumed, three to four units would be produced.[49] A freeze wall is first constructed to isolate the processing area from surrounding groundwater. 2,000 feet (610 m) wells, eight feet apart, are drilled and filled with a circulating super-chilled liquid to cool the ground to −60 °F (−50 °C). Water is then removed from the working zone. Heating and recovery wells are drilled at 40 feet (12 m) intervals within the working zone. Electrical heating elements are lowered into the heating wells and used to heat the kerogen to between 650 °F (340 °C) and 700 °F (370 °C) over a period of approximately four years, slowly converting it into oil and gases, which are then pumped to the surface. Shell believes that it will be possible to recover some 65-70% of the hydrocarbons using this technique.[49] An operation producing 100,000 barrels a day would require a dedicated power generating capacity of 1.2 gigawatts. To maximize the functionality of the freeze walls, adjacent working zones will be developed in succession. This in situ method requires 100% disturbance of the surface, greatly increasing the footprint of extraction operations in comparison to conventional oil and gas drilling. The current test sites are expected to produce in the region of 600 to 1,500 barrels per day (~84 to ~210 t/d).[49] Extensive water use and the risk of groundwater pollution are the technology's greatest challenges.[50]

The EGL Resources Process combines horizontal wells, through which steam is passed, and vertical wells, which provide both vertical heat transfer through refluxing of generated oil and a means to collect and produce the oil. In contrast to the Equity process, the steam circulates through a closed loop, and no fluids are injected into the formation. Horizontal heat transfer from the vertical wells is similar to that in the Shell ICP, and a similar quality of oil is expected. American Shale Oil Corporation (former EGL Oil Shale, a subsidiary of EGL Resources) is leasing a 160 acres (650,000 m2) test tract in the Piceance Basin from the United States Bureau of Land Management to test this technology.[51][52]
In Independent Energy Partners' Geothermic Fuels Cells Process (IEP GFC), a high-temperature stack of fuel cells is placed in the formation to heat the ground. During an initial warm-up period, the cells are fueled by an external source of natural gas. Afterwards, the process is able to fuel itself using gases liberated by its own waste heat. The formation is fractured by rising fluid pressure in the heated zone. Alternatively, the formation can be pre-fractured to enhance the flow of hydrocarbons between heating and producing wells. The company asserts a ratio of approximately 18 units of energy produced per unit used, when primary recovery is combined with gasification of the residual char and use of the resulting synthetic gas.[28]
Externally generated hot gas
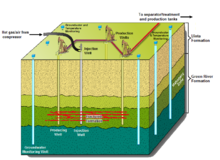
The in situ technologies that use externally-heated gases to decompose oil shale include the Chevron CRUSH process, the Petro Probe process, and Mountain West Energy's In Situ Gas Extraction (MWE IGE) technology. Chevron Corporation and Los Alamos National Laboratory formed a joint research project in 2006 to develop the Chevron CRUSH process. The project is investigating whether heated carbon dioxide can be injected into the formation to decompose the rock. The carbon dioxide would be injected via conventionally-drilled wells and then exposed to the formation via a series of horizontal fractures in which it circulates. The shale oil would then be brought up in conventional vertical oil wells. This method is based on research and trials carried out in the 1950s by Sinclair Oil, which developed a processing method using existing and induced fractures between vertical wells. Continental Oil (now ConocoPhillips) and the University of Akron demonstrated the process; patents were issued that demonstrated the usefulness of carbon dioxide as a heat carrier.[53]
Petro Probe, a subsidiary of Earth Science Search, has described a process which involves injecting super-heated air into wells drilled into the oil shale formation. The super-heated air mixes and melts the shale; its hydrocarbons are transported to the surface in a gaseous state. At the surface, the gas is cooled and the condensate is collected. The remaining gas is re-used to heat the air and is injected back into the formation along with other waste products, thereby minimizing the process's environmental impact.[28] Mountain West Energy's technology (also known as In Situ Vapor Extraction) uses similar principles. A high-temperature gas is injected into the oil shale formation to decompose the oil shale. After it has swept the oil vapors to the surface, where they are condensed and separated, the gas is recirculated.[28][54]
ExxonMobil Electrofrac
ExxonMobil, which has been involved in oil shale development since the 1960s, is focusing on its own in situ technology. A series of hydraulic fractures is created in the rock and an electrically-conductive heating fluid is injected into the formation. The shale oil is then extracted by separate dedicated production wells. The most likely method involves horizontal wells which have been hydraulically fractured along the vertical axis. These wells are placed in a parallel row with a second horizontal well intersecting them at their toe. This will allow opposing electrical charges to be applied at either end. The Electrofrac method has been tested in laboratories and test sites are being considered for a field trial.[28]
Volumetric heating
The concept of volumetric heating by radio waves (radio frequency processing) of oil shale was developed at the Illinois Institute of Technology during the late 1970s. The concept was to heat modest volumes of oil shale, using vertical electrode arrays. Deeper large volumes could be processed at slower heating rates over time. The technology was developed later by Lawrence Livermore National Laboratory, and by several other inventors. This concept was based on the use of installations spaced at tens of meters that would heat sizeable quantities of deep oil shale very slowly. The concept presumed a radio frequency at which the skin depth is many tens of meters, thereby overcoming the thermal diffusion times needed for conductive heating.[2][7] Microwave heating technologies are based on the same principles as radio wave heating, although it is believed that radio wave heating is an improvement over microwave heating because the energy can penetrate farther into the formation.[55] Radio frequency processing is being tested by Raytheon Corporation, while Global Resource Corporation is testing microwave heating.[55][56] Electro-Petroleum proposes electrically enhanced oil recovery for heating oil shale and generating shale oil. Direct current is passed between cathodes in producing wells and anodes located either at the surface or at depth in other wells. The passage of the current through the formation results in resistive Joule heating. This process has increased production from heavy oil fields in short-term tests.[28][57]
Economics

During the early 20th century, the petroleum industry expanded. Since then, the various attempts to develop oil shale deposits have succeeded only when the cost of shale-oil production in a given region is lower than the price of petroleum or its other substitutes. According to a survey conducted by the RAND Corporation, the cost of producing a barrel of oil at a surface retorting complex in the United States (comprising a mine, retorting plant, upgrading plant, supporting utilities, and spent shale reclamation), would range between US$70–95 ($440–600/m3, adjusted to 2005 values). This estimate considers varying levels of kerogen quality and extraction efficiency. In order for the operation to be profitable, the price of petroleum would need to remain above these levels. The analysis discusses the expectation that processing costs would drop after the complex was established. The hypothetical unit would see a cost reduction of 35–70% after its first 500 million barrels (79×106 m3) were produced. Assuming an increase in output of 25 thousand barrels per day (4.0×103 m3/d) during each year after the start of commercial production, the costs would then be expected to decline to $35–48 per barrel ($220–300/m3) within 12 years. After achieving the milestone of 1 billion barrels (160×106 m3), its costs would decline further to $30–40 per barrel ($190–250/m3).[58][59] A comparison of the proposed United States oil shale industry to the Alberta tar-sands industry has been drawn (the latter enterprise generated over one million barrels of oil per day in late 2007), stating that "the first-generation facility is the hardest, both technically and economically".[60][61]
Royal Dutch Shell has announced that its in situ extraction technology in Colorado would realize a profit when crude oil prices are higher than $30 per barrel ($190/m3), while other technologies at full-scale production assert profitability at oil prices even lower than $20 per barrel ($130/m3).[11][62][63][64] To increase the efficiency of oil shale retorting, researchers have proposed and tested several co-pyrolysis processes, in which other materials such as wood or waste plastics are retorted along with the oil shale.[65][66][67][68][69]
Some observers have compared shale-oil production unfavorably with other unconventional oil technologies, arguing that liquefaction of coal costs less than shale oil extraction, produces more oil, and creates fewer environmental impacts. In 1972, the journal Pétrole Informations (ISSN 0755-561X) noted that one ton of coal yielded 650 liters (170 U.S. gal; 140 imp gal) of oil while one ton of oil shale yielded only 150 liters (40 U.S. gal; 33 imp gal) of shale oil.[32]
A critical measure of the viability of oil shale as an energy source lies in the ratio of the energy produced by the shale to the energy used in its mining and processing, a ratio known as "Energy Returned on Energy Invested" (EROEI). A 1984 study estimated the EROEI of the various known oil shale deposits as varying between 0.7–13.3.[70] Royal Dutch Shell has reported an EROEI of three to four on its in situ development, the Mahogany Research Project.[49][62][71] The water needed in the extraction process offers an additional economic consideration: this may pose a problem in areas with water scarcity.
Environmental considerations
Oil shale mining involves a number of environmental impacts, more pronounced in surface mining than in underground mining. They include acid drainage induced by the sudden rapid exposure and subsequent oxidation of formerly buried materials, the introduction of metals into surface water and groundwater, increased erosion, sulfur gas emissions, and air pollution caused by the production of particulates during processing, transport, and support activities.[7][72]
Oil shale extraction can damage the biological and recreational value of land and the ecosystem in the mining area. Combustion and thermal processing generate waste material. In addition, the atmospheric emissions from oil shale processing and combustion include carbon dioxide, a greenhouse gas. Environmentalists oppose production and usage of oil shale, as it generates even more greenhouse gases than conventional fossil fuels.[73] Section 526 of the Energy Independence And Security Act prohibits United States government agencies from buying oil produced by processes that produce more greenhouse gas emissions than traditional petroleum extraction.[74][75] Experimental in situ conversion processes may reduce some of these impacts, but at the same time they may cause other problems, including groundwater pollution. Developing carbon capture and storage technologies may reduce the processes' carbon footprint.[76]
Some commentators have expressed concerns over the oil shale industry's use of water. Depending on technology, above-ground retorting uses between one and five barrels of water per barrel of produced shale-oil.[58][77][78][79] A 2007 programmatic environmental impact statement issued by the United States Bureau of Land Management stated that surface mining and retort operations produce two to ten US gallons (1.5–8 imperial gallons or 8–38 L) of wastewater per tonne of processed oil shale.[77] In situ processing, according to one estimate, uses about one-tenth as much water.[80] Water consumption is a particularly sensitive issue in arid regions, such as the western US and Israel's Negev Desert, where there are plans to expand the oil shale industry despite water shortages.[81]
Environmental organizations, including Greenpeace, have mounted strong protests against the oil shale industry. In one instance, Queensland Energy Resources put the proposed Stuart Oil Shale Project in Australia on hold in 2004.[7][82][83]
See also
References
- ^ a b c d e Koel, Mihkel (1999). "Estonian oil shale". Oil Shale. A Scientific-Technical Journal (Extra). Estonian Academy Publishers. ISSN 0208-189X. Retrieved 2007-07-21.
- ^ a b c d e f g h i j k l m
Burnham, Alan K.; McConaghy, James R. (2006-10-16). "Comparison of the acceptability of various oil shale processes" (PDF). Golden: 26th Oil shale symposium. UCRL-CONF-226717. Retrieved 2007-06-23.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d e f
Qian, Jialin (2006-11-07). World oil shale retorting technologies (PDF). Amman, Jordan: International Oil Shale Conference. Retrieved 2007-06-29.
{{cite conference}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "Oil Shale Activities". United States Department of Energy. Retrieved 2007-10-20.
- ^ Brendow, K. (2003). "Global oil shale issues and perspectives. Synthesis of the Symposium on Oil Shale. 18-19 November, Tallinn" (PDF). Oil Shale. A Scientific-Technical Journal. 20 (1). Estonian Academy Publishers: 81–92. ISSN 0208-189X. Retrieved 2007-07-21.
- ^
Hamarneh, Yousef (1998; 2006). "Oil Shale Resources Development In Jordan" (PDF). Amman: Natural Resources Authority of Jordan. Retrieved 2007-06-16.
{{cite journal}}: Check date values in:|date=(help); Cite journal requires|journal=(help) - ^ a b c d
A.K. Burnham (2003-08-20). "Slow Radio-Frequency Processing of Large Oil Shale Volumes to Produce Petroleum-like Shale Oil" (PDF). Lawrence Livermore National Laboratory. UCRL-ID-155045. Retrieved 2007-06-28.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c d
"An Assessment of Oil Shale Technologies" (PDF). June 1980. NTIS order #PB80-210115. Retrieved 2007-11-03.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b c Mölder, Leevi (2004). "Estonian Oil Shale Retorting Industry at a Crossroads" (PDF). Oil Shale. A Scientific-Technical Journal. 21 (2). Estonian Academy Publishers: 97–98. ISSN 0208-189X. Retrieved 2007-06-23.
- ^ a b
Soone, Jüri (2006-11-07). Environmentally sustainable use of energy and chemical potential of oil shale (PDF). Amman, Jordan: International Oil Shale Conference. Retrieved 2007-06-29.
{{cite conference}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b c d e f g h
"Strategic significance of America's oil shale resource. Volume II: Oil shale resources, technology and economics" (PDF). Office of Deputy Assistant Secretary for Petroleum Reserves; Office of Naval Petroleum and Oil Shale Reserves; United States Department of Energy. 2004. Retrieved 2007-06-23.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Purga, Jaanus (2004). "Today's rainbow ends in Fushun" (PDF). Oil Shale. A Scientific-Technical Journal. 21 (4). Estonian Academy Publishers: 269–272. ISSN 0208-189X. Retrieved 2007-06-23.
- ^
Committee on Production Technologies for Liquid Transportation Fuels, Energy Engineering Board, National Research Council (1990). Fuels to drive our future. National Academy Press. p. 183. ISBN 978-0-309-08645-5. Retrieved 2008-05-04.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "Shale Technologies". Shale Technologies. Retrieved 2007-06-24.
- ^ "Shale Technologies, LLC". Process Register, Inc. Retrieved 2008-08-15.
- ^
"Notes to financial statements to the U.S. Securities and Exchange Commission". Regent Energy Corporation. 2001-02-14. 10QSB SEC. Retrieved 2008-08-15.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Liive, Sandor (2007). "Oil Shale Energetics in Estonia" (PDF). Oil Shale. A Scientific-Technical Journal. 24 (1). Estonian Academy Publishers: 1–4. ISSN 0208-189X. Retrieved 2007-06-24.
- ^
"Annual Report 2007/2008" (PDF). Eesti Energia. 2008. Retrieved 2008-08-15.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b "Jordan Oil Shale Project". Omar Al-Ayed, Balqa Applied University. 2008. Retrieved 2008-08-15.
- ^ "New shale oil line for VKG Oil AS" (PDF). Rintekno Newsletter. 20. Rintekno Oy. 2006. Retrieved 2007-06-24.
- ^ US 5366596Dry thermal processor.
- ^ a b "UMATAC and the Alberta Taciuk Process". AECOM Technology Group. Retrieved 2008-08-13.
- ^ Chandler, Graham (2006). "US eyes Alberta as model for developing oil shale" (PDF). Alberta Oil. 2 (4): 16–18. Retrieved 2007-06-24.
- ^ "Main project description". Jordan Energy and Mining Limited. Retrieved 2008-05-10.
- ^ Lee, Sunggyu (1991). Oil Shale Technology. CRC Press. pp. 117−118. ISBN 0849346150. Retrieved 2008-05-11.
- ^
Dyni, John R. (2006). "Geology and resources of some world oil shale deposits. Scientific Investigations Report 2005–5294" (PDF). United States Department of the Interior; United States Geological Survey. Retrieved 2007-07-09.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Sandler, Neal (2006). "Israel assesses new oil shale technology" (PDF). Energy Economist (297). Platts. Retrieved 2007-11-11.
- ^ a b c d e f g h i j
"Secure Fuels from Domestic Resources: The Continuing Evolution of America's Oil Shale and Tar Sands Industries" (PDF). United States Department of Energy. 2007: 1–68. Retrieved 2007-07-11.
{{cite journal}}: Cite journal requires|journal=(help) - ^
Patten, James W. (2007). "Red-Leaf Resources. Presentation at the Utah Energy Summit" (PDF). Utah Energy Summit. Retrieved 2007-11-11.
{{cite journal}}: Cite journal requires|journal=(help) - ^
"27th Oil Shale Symposium Proceedings". Colorado School of Mines Press. 2007.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b
"Draft Oil Shale and Tar Sands Resource Management Plan Amendments to Address Land Use Allocations in Colorado, Utah, and Wyoming and Programmatic Environmental Impact Statement. Appendix A: Oil Shale Development Background and Technology Overview" (PDF). Argonne National Laboratory. 2007-12-07: 54 − 55. Retrieved 2008-05-11.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b
Laherrère, Jean (2005). "Review on oil shale data" (PDF). Hubbert Peak. Retrieved 2007-06-17.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "OSEC's Process: A Proven Technology". Oil Shale Exploration Company, LLC. Retrieved 2007-06-24.
- ^ "Oil Shale Exploration Company Signs Agreement with Affiliates of Petrobras and Mitsui". Oil Voice. 2008-06-10. Retrieved 2008-06-21.
- ^ "Xtract Ltd". Xtract Energy Plc. 2007. Retrieved 2008-05-01.
- ^
Weil, S. A.; Feldkirchner, H. L.; Punwani, D. V.; Janka, J. C. (1979-01-01). "IGT HYTORT Process for hydrogen retorting of Devonian oil shales". Institute of Gas Technology,Chicago, IL. CONF-790571-3.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Rex, R.; Janka, J. C.; Knowlton, T. (1984). Cold Flow Model Testing of the Hytort Process Retort Design. 17th Oil Shale Symposium. Colorado School of Mines Press. pp. 17–36.
- ^
Ennis, D.L. (2006-08-15). "Oil Shale—An Investment We Can't Afford". California Chronicle. Retrieved 2008-05-01.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Kök, M. V.; Guner, G.; Suat Bağci, A. (2008). "Application of EOR techniques for oil shale fields (in-situ combustion approach)" (PDF). Oil Shale. A Scientific-Technical Journal. 25 (2). Estonian Academy Publishers: 217–225. doi:10.3176/oil.2008.2.04. ISSN 0208-189X. Retrieved 2008-06-07.
- ^ Dougan, P.M.; Dockter, L. (1981). BX In Situ Oil Shale Project. 14th Oil Shale Symposium. Colorado School of Mines Press. pp. 118–127.
- ^ a b Tyner, C.E.; Parrish, R.L.; Major, B.H.; Lekas, J.M. (1982). Sandia/Geokinetics Retort 23: A Horizontal In Situ Retorting Experiment. 15th Oil shale Symposium Proceedings. Colorado School of Mines Press. pp. 370–384.
- ^
Costimiris, E.C. (1982). "Investigation of the Geokinetics horizontal in-situ oil-shale-retorting process. Annual Report 1981". Geokinetics, Inc.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Lombard, D.B.; Carpenter, H.C. (1967). "Recovering Oil by Retorting a Nuclear Chimney in Oil Shale". Journal of Petroleum Technology (19). Society of Petroleum Engineers: 727–734.
- ^ Gregg, M.L.; Campbell, J.H. (1980). Sweep efficiency modeling of modified in situ retorts. 13th Oil shale Symposium Proceedings. Colorado School of Mines Press. pp. 87–100.
- ^
Bickel, T.C. (1984). "Data Acquisition and Analysis of Occidental Vertical Modified In Situ Retorts 7 and 8, Sandia Report SAND83-2649".
{{cite journal}}: Cite journal requires|journal=(help) - ^ Berry, K.L.; Hutson, R.L.; Sterrett, J.S.; Knepper, J.C. (1982). Modified In-Situ Retorting Results of Two Field Retorts. 15th Oil shale Symposium Proceedings. Colorado School of Mines Press. pp. 385–396.
- ^ "Integrated in situ shale oil and mineral recovery process". FreePatentsOnline. 1981-08-25. United States Patent 4285547. Retrieved 2007-11-11.
- ^ "Oil Shale Projects". Agapito Associates, Inc. Retrieved 2008-08-13.
- ^ a b c d
"Oil Shale Test Project. Oil Shale Research and Development Project" (PDF). Shell Frontier Oil and Gas Inc. 2006-02-15. Retrieved 2007-06-30.
{{cite journal}}: Cite journal requires|journal=(help) - ^
Jon Birger (2007-11-01). "Oil shale may finally have its moment". Fortune. Retrieved 2007-11-17.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "Innovation in Shale Technology". EGL Shale. 2006-11-16. Retrieved 2007-07-06.
- ^
"Plan of Operation for Oil Shale Research, Development and Demonstration (R,D/D) Tract" (PDF). E.G.L. Resources, Inc. 2006-02-15. Retrieved 2008-05-01.
{{cite journal}}: Cite journal requires|journal=(help) - ^
"Oil Shale Research, Development & Demonstration Project. Plan of Operation" (PDF). Chevron USA Inc. 2006-02-15. Retrieved 2008-05-01.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "MWE's In-situ Vapor Extraction Technology (IVE)". Mountain West Energy LLC. Retrieved 2008-05-02.
- ^ a b Megan Harris (2007-07-20). "Oil from shale could meet need". United Press International. Retrieved 2007-08-19.
- ^ "Global Resource Reports Progress on Oil Shale Conversion Process". Downstream Today. 2007-03-09. Retrieved 2008-05-31.
- ^
"Electrically enhanced oil recovery using DC technology". Electro-Petroleum, Inc. Retrieved 2008-05-01.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b
Bartis, James T.; LaTourrette, Tom; Dixon, Lloyd; Peterson, D.J.; Cecchine, Gary (2005). "Oil Shale Development in the United States. Prospects and Policy Issues. Prepared for the National Energy Technology Laboratory of the [[United States Department of Energy]]" (PDF). The RAND Corporation. ISBN 978-0-8330-3848-7. Retrieved 2007-06-29.
{{cite journal}}: Cite journal requires|journal=(help); URL–wikilink conflict (help) - ^
"A study on the EU oil shale industry viewed in the light of the Estonian experience. A report by EASAC to the Committee on Industry, Research and Energy of the European Parliament" (PDF). European Academies Science Advisory Council. May 2007. Retrieved 2007-11-25.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "A Reporter at Large:Unconventional Crude". The New Yorker. 2007-11-12. Retrieved 2008-03-31.
- ^ "Is Oil Shale The Answer To America's Peak-Oil Challenge?" (PDF). United States Department of Energy. 2008-02-08. Retrieved 2008-03-31.
- ^ a b
Seebach, Linda (2005-09-02). "Shell's ingenious approach to oil shale is pretty slick". Rocky Mountain News. Retrieved 2007-06-02.
{{cite news}}: Italic or bold markup not allowed in:|publisher=(help) - ^ Schmidt, S. J. (2003). "New directions for shale oil:path to a secure new oil supply well into this century: on the example of Australia" (PDF). Oil Shale. A Scientific-Technical Journal. 20 (3). Estonian Academy Publishers: 333–346. ISSN 0208-189X. Retrieved 2007-06-02.
- ^ Krauss, Leah (2006-11-07). "Analysis: Israel sees shale replacing oil". United Press International. Retrieved 2007-07-29.
- ^ Tiikma, Laine; Johannes, Ille; Pryadka, Natalja (2002). "Co-pyrolysis of waste plastics with oil shale". Proceedings. Symposium on Oil Shale 2002, Tallinn, Estonia: 76.
- ^ Tiikma, Laine; Johannes, Ille; Luik, Hans (March 2006). "Fixation of chlorine evolved in pyrolysis of PVC waste by Estonian oil shales" (PDF). Journal of Analytical and Applied Pyrolysis. 75 (2): 205–210. doi:10.1016/j.jaap.2005.06.001. Retrieved 2007-10-20.
- ^ Veski, R.; Palu, V.; Kruusement, K. (2006). "Co-liquefaction of kukersite oil shale and pine wood in supercritical water" (PDF). Oil Shale. A Scientific-Technical Journal. 23 (3). Estonian Academy Publishers: 236–248. ISSN 0208-189X. Retrieved 2007-06-16.
- ^ Aboulkas, A.; El Harfi, K.; El Bouadili, A.; Benchanaa, M.; Mokhlisse, A.; Outzourit, A. (2007). "Kinetics of co-pyrolysis of Tarfaya (Morocco) oil shale with high-density polyethylene" (PDF). Oil Shale. A Scientific-Technical Journal. 24 (1). Estonian Academy Publishers: 15–33. ISSN 0208-189X. Retrieved 2007-06-16.
- ^
Ozdemir, M.; A. Akar, A. Aydoğan, E. Kalafatoglu; E. Ekinci (2006-11-07). Copyrolysis of Goynuk oil shale and thermoplastics (PDF). Amman, Jordan: International Oil Shale Conference. Retrieved 2007-06-29.
{{cite conference}}: CS1 maint: multiple names: authors list (link) - ^
Cleveland, Cutler J.; Costanza, Robert; Hall, Charles A. S.; Kaufmann, Robert (1984-08-31). "Energy and the U.S. Economy: A Biophysical Perspective" (PDF). Science. 225 (4665). American Association for the Advancement of Science: 890–897. doi:10.1126/science.225.4665.890. ISSN 0036-8075. PMID 17779848. Retrieved 2007-08-28.
{{cite journal}}: CS1 maint: date and year (link) - ^ Reiss, Spencer (2005-12-13). "Tapping the Rock Field". WIRED Magazine. Retrieved 2007-08-27.
- ^
"Environmental Impacts from Mining" (PDF). US Office of Surface Mining Reclamation and Enforcement. 2006-08-02. Retrieved 2008-03-29.
{{cite journal}}: Cite journal requires|journal=(help) - ^
"Driving It Home. Choosing the Right Path for Fueling North America's Transportation Future" (PDF). Natural Resources Defense Council. June 2007. Retrieved 2008-04-19.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Kosich, Dorothy (2008-04-11). "Repeal sought for ban on U.S. Govt. use of CTL, oil shale, tar sands-generated fuel". Moneyweb Holdings. Retrieved 2008-05-27.
- ^
Bloom David I, Waldron Roger, Layton Duane W, Patrick Roger W (2008-03-04). "United States: Energy Independence And Security Act Provision Poses Major Problems For Synthetic And Alternative Fuels". Retrieved 2008-05-27.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^
Bartis, Jim (2006-10-26). "Unconventional Liquid Fuels Overview. 2006 Boston World Oil Conference" (PDF). Association for the Study of Peak Oil & Gas - USA. Retrieved 2007-06-28.
{{cite journal}}: Cite journal requires|journal=(help) - ^ a b "Draft Oil Shale and Tar Sands Resource Management Plan Amendments to Address Land Use Allocations in Colorado, Utah, and Wyoming and Programmatic Environmental Impact Statement. Volume 2" (PDF). Argonne National Laboratory. 2007-12-07. p. 36. Retrieved 2008-03-31.
- ^ Luken, Larry (2005-07-09). "Oil Shale Myths". Shale Oil Information Center. Retrieved 2008-04-01.
- ^ "Critics charge energy, water needs of oil shale could harm environment". U.S. Water News Online. July 2007. Retrieved 2008-04-01.
- ^
"Hopes for shale oil are revived". worldoil.com. 2005. Retrieved 2008-04-01.
{{cite web}}: Unknown parameter|month=ignored (help) - ^
"Oil-shale 'rush' is sparking concern". Deseret Morning News. 2008-03-22. Retrieved 2008-03-31.
{{cite web}}: Italic or bold markup not allowed in:|publisher=(help) - ^ "Climate-changing shale oil industry stopped". Greenpeace Australia Pacific. 2005-03-03. Retrieved 2007-06-28.
- ^ "Greenpeace happy with part closure of shale oil plant". Australian Broadcasting Corporation. 2004-07-22. Retrieved 2008-05-19.
External links
- Oil Shale and Tar Sands Draft Programmatic Environmental Impact Statement (EIS) Concerning potential leases of Federal oil sands lands in Utah and oil shale lands in Utah, Wyoming, and Colorado
