Etard reaction
The Étard reaction is a name reaction in organic chemistry that was named after its discoverer Alexandre Léon Étard (1852–1910).
Overview reaction
The carbon atom of an alkyl-substituted phenyl radical ( toluene ) is oxidized with chromium (VI) oxide dichloride to form the corresponding aldehyde . In the following example, a methyl-substituted phenyl radical reacts to form benzaldehyde .
Analogously, a methyl substituent on a cycloalkyl ring (e.g. in methylcyclohexane) can also be oxidized to a formyl group.
Reaction mechanism
The following mechanism is suggested by Ji Jack Li. In the first step, an ene reaction takes place in which toluene 1 is reacted with chromium (VI) oxide dichloride. This produces the red to brown étard complex 2 .
This breaks down to compound 3 through a [2 + 3] sigmatropic rearrangement reaction . In this case, reducing conditions are necessary in order to prevent oxidation to the carboxylic acid . Another rearrangement reaction produces benzaldehyde 4 .
Historical background
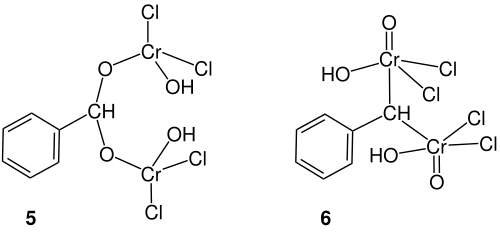
The reaction mechanism of the Etard reaction has not yet been fully elucidated. The mechanism described by Ji Jack Li is only one of many proposed. After Étard formulated his mechanism in 1881, the structure of the resulting chelate complex was discussed above all. This gave rise to the idea of the existence of a chromium (IV) chelate complex 5 .
Rhode used the debate in 1901 as an opportunity to investigate different oxidation states of chromium in the context of the Etard reaction. He formulated the chelate complex 6 . In 1951, Tillotson and Houston reacted two moles of the chromium compound with only one mole of the organic compounds. Even if this formula was already known from other reactions, it is difficult to accept a chromium (VI) compound with a coordination number of five.
Slack and Waters introduced the approach of a radical reaction in the discussion, whereby the addition reaction of the first chromium (VI) oxide dichloride molecule represents the slow and therefore rate-determining step. Thus many different mechanisms emerged, some of which are more likely than others. The one proposed by Ji Jack Li is one of the most recent. A similar number of possibilities have been proposed for the breakdown of the complex, the influence of the solvent, and other details. This resulted in many variants of the Etard reaction, which significantly increased the substrate scope. The reaction is disturbed by rearrangement reactions, so that, for example, ketones are formed as by-products .
Practical meaning
The osmophoric aldehyde group is often characterized by pleasant smells . Aldehydes are very reactive and are therefore often used in addition , condensation or polymerization reactions. For example, lower aldehydes are often converted into plastics and synthetic resins . Higher aldehydes are processed into fragrances , such as perfumes . The smell of the benzaldehyde obtained in the example is often compared with the smell of marzipan or almonds . Often used as a solvent in the laboratory, it is used in the manufacture of cinnamic acid and pharmaceuticals . Triphenylmethane dyes are also synthesized from benzaldehyde. They are mainly used in printing technology .
Further syntheses
Large-scale process for the preparation of aldehydes
Laboratory process for the preparation of aldehydes
literature
- Jie Jack Li: Name reactions, a collection of detailed reaction mechanism. Vol 1. Springer 2002 , p. 113. ISBN 3-540-43024-5 .
- Hermann Römpp, Jürgen Falbe, Eckard Amelingmeier: Römpp-Lexikon Chemie , Vol 9. Thieme-Verlag, Stuttgart 1999 . ISBN 3-13-107830-8 .
- Michael B. Smith: March's advanced organic chemistry. Reactions, mechanism, and structure , Vol 7. John Wiley & Sons, New Jersey 2013 , pp. 1479-1450. ISBN 978-0-470-46259-1 .
Individual evidence
- ^ A. Hassner, I. Namboothiri: Organic Syntheses Based on Name Reactions, 3rd edition, Elsevier, 2012, p. 145, ISBN 978-0-08-096630-4 .
- ^ Owen H. Wheeler: Étard Reaction: I. Its Scope and Limitation with Substituted Toluenes. In: Canadian Journal of Chemistry . 36 (4), 1958 , pp. 667-671, doi : 10.1139 / v58-093 .
- ↑ Ileana Necsoiu, AT Balaban, I. Pascaru, Elvira Sliam, M. Elian, CD Nenitzescu: The mechanism of the etard reaction. In: Tetrahedron. 19, No. 7, 1963 , pp. 1133-1142, doi: 10.1016 / S0040-4020 (01) 98572-2 .
- ↑ CN Renţea, I. Necşoiu, M. Renţes, A. Ghenciulescu, CD Nenitzescu: Étard reaction — III: Oxidation of N-propylbenzene and methylcyclohexane with chromyl chloride. In: Tetrahedron. 22, No. 10, 1966 , pp. 3501-3513, doi: 10.1016 / S0040-4020 (01) 92538-4 .
- ↑ CN Renţea, I. Necşoiu, A. Ghenciulescu, CD Nenitzescu, V. Przemetchi: Etard reaction — II: Structure of the chromyl chloride complexes of Phenylmethanes. In: Tetrahedron. 22, No. 9, 1966 , pp. 3037-3045, DOI: 10.1016 / S0040-4020 (01) 82283-3 .
- ^ Winslow H. Hartford, Marc Darrin: The Chemistry Of Chromyl Compounds. In: Chemical Reviews , 58, 1958 , pp. 1-61, doi : 10.1021 / cr50019a001 .
- ↑ Kenneth B. Wiberg, Brian Marshall, Gordon Foster: Some observations on the étard reaction. In: Tetrahedron Letters. 3, No. 8, 1962 , pp. 345-348, doi : 10.1016 / S0040-4039 (00) 70878-1 .