2,6-dichlorophenylacetonitrile
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 2,6-dichlorophenylacetonitrile | ||||||||||||||||||
| other names |
2,6-dichlorobenzyl cyanide |
||||||||||||||||||
| Molecular formula | C 8 H 5 Cl 2 N | ||||||||||||||||||
| Brief description |
off-white crystal powder |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 186.04 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.4274 g cm −3 at 20 ° C |
||||||||||||||||||
| Melting point |
74-77 ° C |
||||||||||||||||||
| solubility |
in methanol |
||||||||||||||||||
| Refractive index |
1.5690 (25 ° C, 589 nm) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
2,6-dichlorophenylacetonitrile is a substituted acetonitrile which has a phenyl group substituted in the 2- and in the 6-position by a chlorine atom . The compound is the starting material for chemical intermediates and pharmaceuticals , such as. B. guanfacine , from the group of antisympathotonics , which is also used in particular for the treatment of attention deficit / hyperactivity disorder ADHD.
Manufacturing
The production of 2,6-dichlorophenylacetonitrile proceeds in the sense of a Kolbe nitrile synthesis , 2,6-dichlorobenzyl chloride (by chlorination of 2,6-dichlorotoluene according to the SSS rule ) with alkali cyanides, such as. B. potassium cyanide is implemented in the presence of the phase transfer catalyst 18-crown-6 (yield 95%).
properties
2,6-dichlorophenylacetonitrile is a white, odorless solid that dissolves in alcohols such as. B. methanol, dissolves.
Applications
The reduction (chemistry) of 2,6-dichlorobenzyl cyanide with diisobutylaluminum hydride DIBAL provides 2,6-dichlorophenylacetaldehyde in useful yields as the starting material for the herbicide diclobenil, which is no longer approved in the EU .
With 2,6-dichlorophenylacetonitrile as a starting material, fused bicyclic heteroaromatics were prepared which, like z. B. the substituted pyrazolo [1,5-a] pyrimidin-2-ones as pharmaceutical active ingredients against autoimmune diseases and cancer , as well as the derivatives of the diazanaphthalene 1,6-naphthyridin-2 (1 H ) -one as tyrosine kinase inhibitors were investigated.
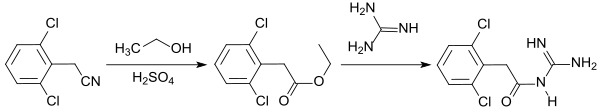
The most important use of 2,6-dichlorophenylacetonitrile is as starting material for the ADHD therapeutic guanfacine . To do this, the nitrile is first converted into the corresponding ethyl ester and reacted with guanidine at room temperature. The product 2,6-dichlorophenylacetylguanidine is obtained in about 70% yield.
Alternatively, 2,6-dichlorophenylacetonitrile is first formylated with ethyl formate on the activated methylene group and then reacted with guanidine hydrochloride to give α- (guanidinomethylene) -2,6-dichlorophenylacetonitrile. The cyano group is split off and rearranged to form 2,6-dichlorophenylacetylguanidine hydrochloride by boiling with dilute hydrochloric acid.
Individual evidence
- ↑ a b c d data sheet 2,6-dichlorophenylacetonitrile from AlfaAesar, accessed on November 28, 2019 ( PDF )(JavaScript required) .
- ^ A b Carl L. Yaws: Thermophysical Properties of Chemicals and Hydrocarbons, 2nd Edition . Elsevier Inc., Oxford, UK 2015, ISBN 978-0-323-28659-6 , pp. 179 .
- ↑ Entry on 2,6-dichlorobenzyl cyanide at TCI Europe, accessed on November 28, 2019.
- ↑ a b Patent WO200705866A2 : Inhibitors of P30 kinase and methods of treating inflammatory disorders. Filed July 18, 2006 , published February 8, 2007 , Applicant: Kalypsys, Inc., Inventors: DL Severance, AJ Borchardt, EMM Gardiner, M. Kahraman.
- ↑ patent US20190330157A1 : Therapeutic compounds. Filed June 3, 2019 , published October 31, 2019 , applicant: Regents of the University of Minnesota, inventor: SS More, R. Vince.
- ↑ AM Thompson, GW Rewcastle, SL Boushelle, BG Hartl, AJ Kraker, GH Lu, BL Batley, RL Panek, HD Showalter, WA Denny: Synthesis and structure-activity relationships of 7-substituted 3- (2,6-dichlorophenyl) -1,6-naphthyridin-2 (1 H ) -ones as selective inhibitors of pp60 (c-src) . In: J. Med. Chem. Volume 43 , no. 16 , 2000, pp. 3134-3147 , doi : 10.1021 / jm000148t .
- ↑ Patent CH479559 : Process for the production of acylguanidines. Applied on September 26, 1967 , published November 28, 1969 , applicant: Dr. A. Wander AG, inventor: JB Bream, CW Picard.
- ↑ Patent CH511816 : Process for the production of acylguanidines. Applied on February 26, 1967 , published October 15, 1971 , applicant: Dr. A. Wander AG, inventor: JB Bream, CW Picard.